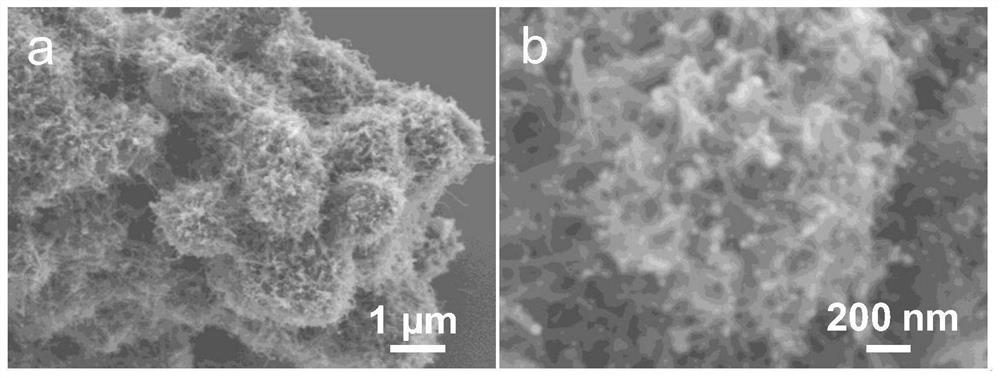
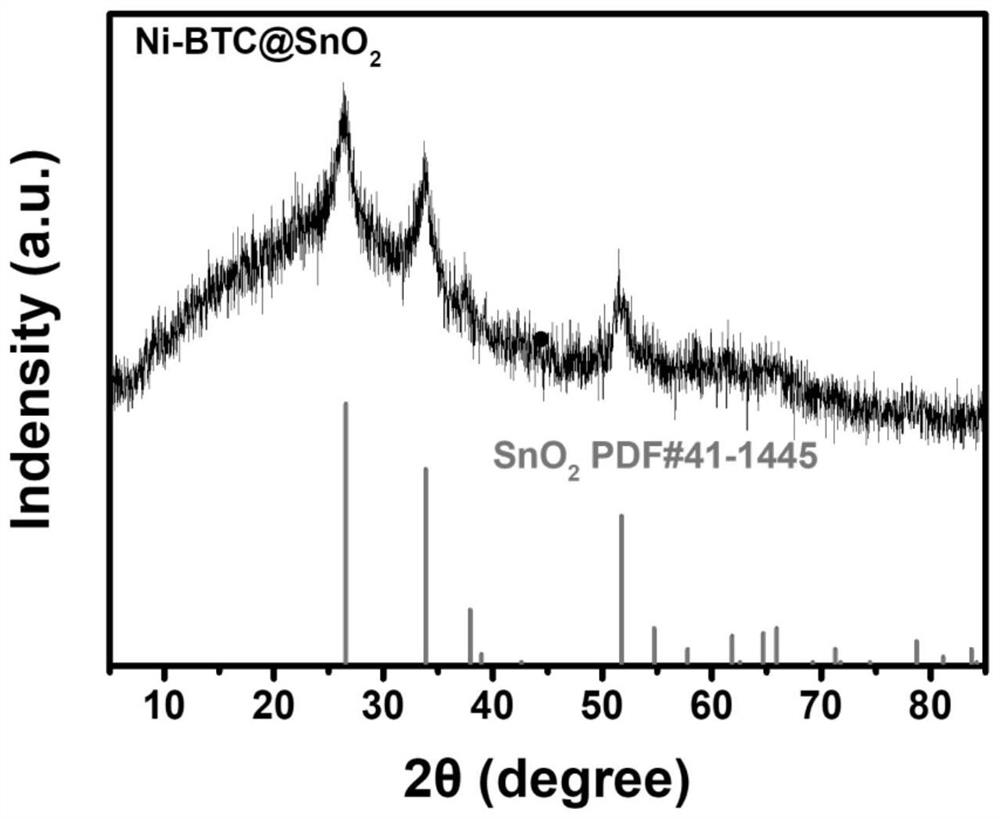
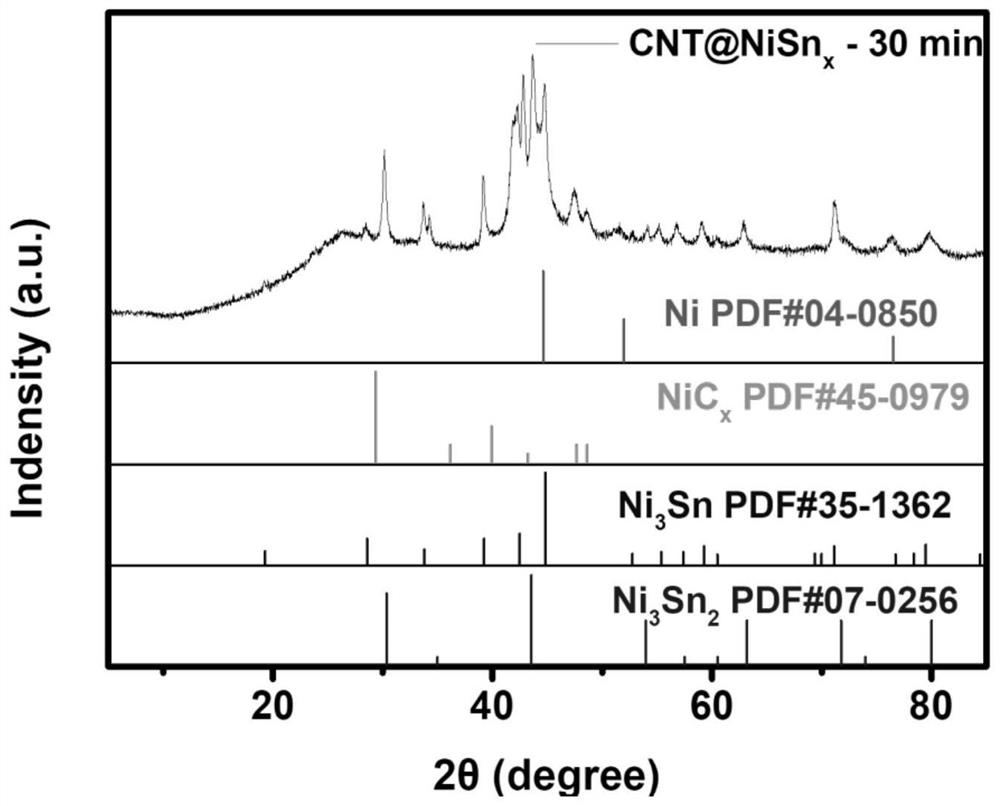
MOF catalytic growth carbon nanotube coated nickel-tin alloy electrode material as well as preparation method and application thereof
A nickel-tin alloy and electrode material technology, applied in chemical instruments and methods, negative electrodes, battery electrodes, etc., can solve the problems of material instability, reduced material cycle stability, and no conductivity, etc. The effect of high stability and capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 1. Hollow SnO 2 Preparation of nanospheres
[0037] SnO with hollow structure synthesized by hydrothermal method 2 nanospheres. In the experiment, put 0.1g of sodium stannate tetrahydrate into a beaker, then add 25mL of deionized water and 15mL of ethanol under magnetic stirring, then add 0.24g of urea into the beaker and stir until completely dissolved. Subsequently, the above solution was transferred to a 100 mL polytetrafluoroethylene-lined stainless steel reactor, and heated in an oven at 150 °C for 15 h for hydrothermal reaction. After cooling to room temperature, the precipitate was separated by centrifugation and washed with deionized water. ℃ dried in a vacuum oven overnight.
[0038] 2. Ni-BTC@SnO 2 preparation of
[0039] 0.05g SnO 2 Pack into and fill the beaker of the 30mL mixed solvent that deionized water: ethanol:DMF=1:1:1 is made into, then add 432mg Ni(NO 3 ) 2 ·6H 2 O, 150mg BTC and 1.5g PVP, stirred vigorously at room temperature until comple...
Embodiment 1
[0043] Put 0.1g of sodium stannate tetrahydrate into a beaker, then add 25mL of deionized water and 15mL of ethanol under magnetic stirring, then add 0.24g of urea into the beaker and stir until completely dissolved. Subsequently, the above solution was transferred to a 100 mL polytetrafluoroethylene-lined stainless steel reactor, and heated in an oven at 150 °C for 15 h for hydrothermal reaction. After cooling to room temperature, the precipitate was separated by centrifugation and washed with deionized water. ℃ dried in a vacuum oven overnight.
[0044] 0.05g SnO 2 Pack into and fill the beaker of the 30mL mixed solvent that deionized water: ethanol:DMF=1:1:1 is made into, then add 432mg Ni(NO 3 ) 2 ·6H 2 O, 150mg BTC and 1.5g PVP, stirred vigorously at room temperature until completely dissolved. Subsequently, the bright green solution obtained above was transferred to a 50 mL polytetrafluoroethylene-lined stainless steel reaction kettle, and heated in an oven at 150 °C...
Embodiment 2
[0048] Put 0.1g of sodium stannate tetrahydrate into a beaker, then add 25mL of deionized water and 15mL of ethanol under magnetic stirring, then add 0.24g of urea into the beaker and stir until completely dissolved. Subsequently, the above solution was transferred to a 100 mL polytetrafluoroethylene-lined stainless steel reactor, and heated in an oven at 150 °C for 15 h for hydrothermal reaction. After cooling to room temperature, the precipitate was separated by centrifugation and washed with deionized water. ℃ dried in a vacuum oven overnight.
[0049] 0.05g SnO 2 Pack into and fill the beaker of the 30mL mixed solvent that deionized water: ethanol:DMF=1:1:1 is made into, then add 432mg Ni(NO 3 ) 2 ·6H 2 O, 150mg BTC and 1.5g PVP, stirred vigorously at room temperature until completely dissolved. Subsequently, the bright green solution obtained above was transferred to a 50 mL polytetrafluoroethylene-lined stainless steel reaction kettle, and heated in an oven at 150 °C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com