Preparation method of N-isobutyrylguanosine
A technology of isobutyryl guanosine and isobutyryl chloride is applied in the field of preparation of N-isobutyryl guanosine, which can solve the problem of low yield, lower product quality, and the number of reactions between isobutyryl chloride and methyl deoxyguanosine Less problems, high yield and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
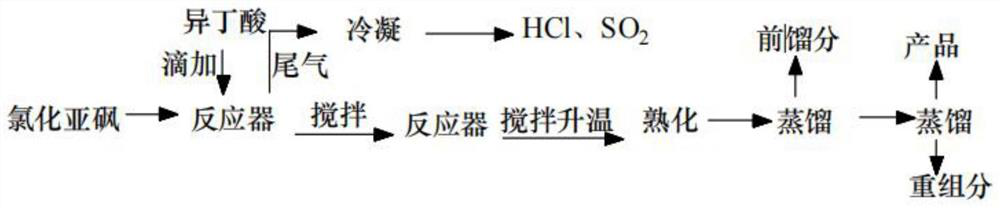
[0032] see figure 2 Shown, S1: Synthesis of isobutyryl chloride
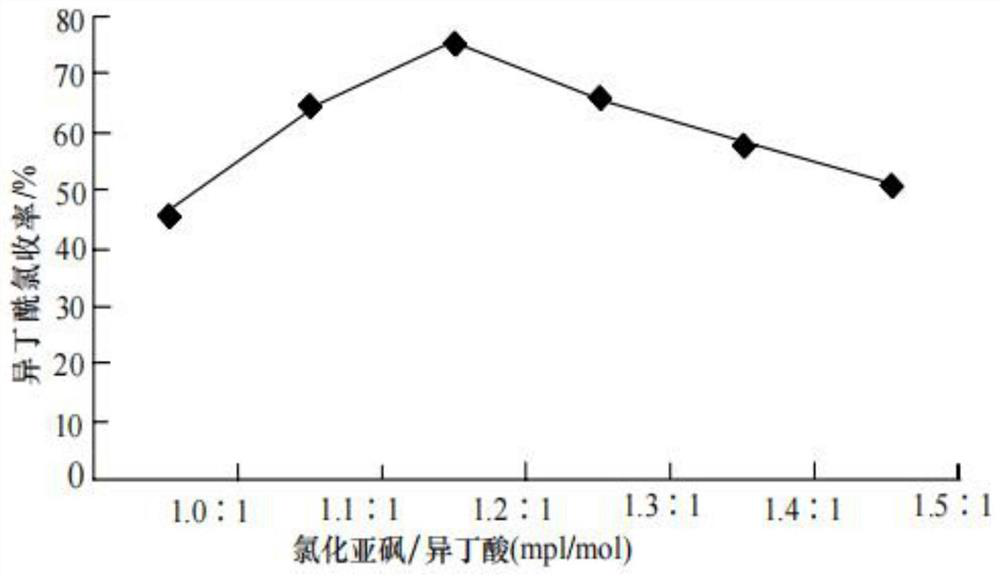
[0033] In a 1000mL reactor with stirrer, constant pressure titration funnel, spherical condenser and thermometer, add 1084g (9.1mol) thionyl chloride, then add 704g (8mol) isobutyric acid in the constant pressure titration funnel , open the water supply valve of the spherical condensation, and start to stir continuously, then add isobutyric acid dropwise under stirring, thionyl chloride and isobutyric acid react at a certain temperature, and the tail gas generated by the reaction is condensed and discharged HCl, SO2, isobutyric acid After the addition of butyric acid is completed, continue to stir and raise the temperature to 80°C, ripen for 40 minutes, and rectify the reaction liquid on the tower. The fraction at ℃ is the synthesized isobutyryl chloride;
[0034] The molecular formula of synthetic isobutyryl chloride is The molecular weight is 106.551, the density is 1.0±0.1g / cm3, the boiling point is 90.9...
Embodiment 2
[0041] S2: Guanosine synthesis
[0042] Put the guanine and the ribose ring into the mixer and mix them, and then connect the guanine and the ribose ring through the β-N9-glycosidic bond to obtain guanosine;
[0043]Guanine is prepared by the following steps:
[0044] (1) In a 100ml double-necked round bottom flask with a distillation device, add 6g of potassium hydroxide, dissolve it with 10ml of water, keep the temperature of the water bath at 60-70°C, and add 35ml of methyl cellosolve under stirring to make it Mix well, then add dropwise 35ml of ether solution containing 5.25g of N-methyl-N-nitroso-p-toluenesulfonamide to this solution, keep the solution under reflux, cool the receiver with an ice bath, and the distillate is diazomethane ether solution;
[0045] (2) Dissolve 0.25g of 2'-deoxynucleoside in 75ml of methanol, keep at 0°C, then add diazomethane ether solution, continue to maintain at 0°C, react for 2h, filter out the white precipitate, wash twice with a small...
Embodiment 3
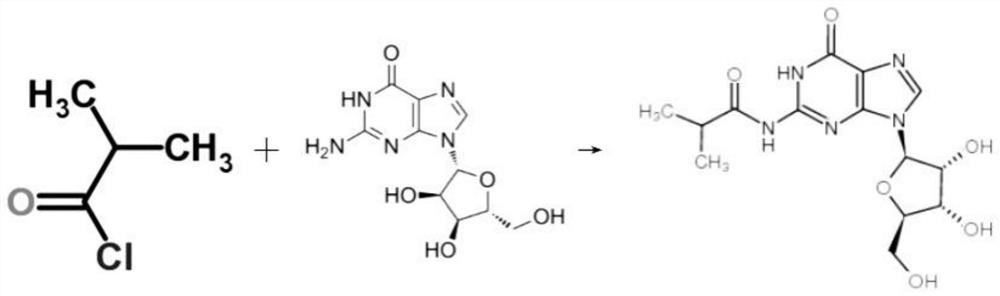
[0049] see figure 1 Shown, S3: N-isobutyrylguanosine synthesis
[0050] S3.1: Add the isobutyryl chloride liquid obtained in step S1 and the methyl deoxyguanosine obtained in step S2 to the reactor for one reaction, the reaction time is 24 hours, the reaction temperature is 40°C, and the internal temperature of the reactor after the reaction down to 0°C;
[0051] S3.2: Then add methanol to the reactor and carry out a secondary reaction. The secondary reaction time is 4 hours, and the reaction temperature is 60°C. After the reaction, the mixed solution is concentrated in a distillation equipment, and the temperature of the distillation equipment is 120°C;
[0052] S3.3: Finally, put the concentrated solution into the reactor, then add the mixed solution of n-ethane and diethyl ether to carry out three reactions, the volume ratio of n-ethane and diethyl ether is 1:1, the three-time reaction time is 12h, and the reaction temperature is 30°C , standing for 2 hours after the reac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com