Anti-SARS-CoV-2 nucleocapsid protein monoclonal antibody as well as preparation method and application thereof
A monoclonal antibody, nucleocapsid protein technology, applied in antiviral immunoglobulin, antibody, immunoglobulin and other directions, can solve the problem of lack of qualified antibody raw materials, achieve short detection cycle, less workload, high throughput Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: the acquisition of SARS-CoV-2 virus N protein hybridoma cell line
[0105] 1) Animal immunity
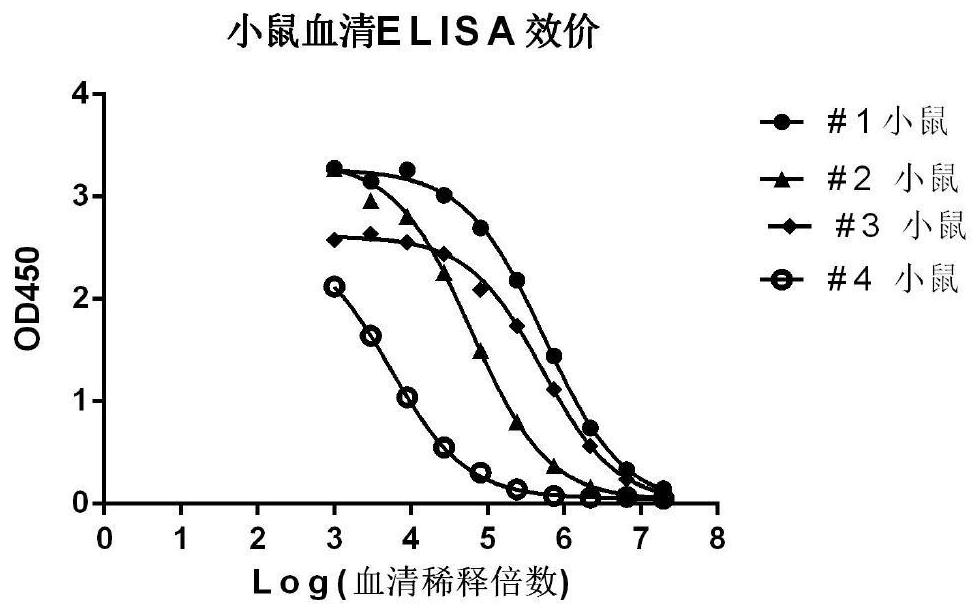
[0106] The antigen is recombinant SARS-CoV-2 virus N protein (Nanjing GenScript Biotechnology Co., Ltd., CatNo.T80103, SEQ ID NO: 1). Female Balb / c mice were subcutaneously immunized with a 1:1 emulsion containing 50 μg of SARS-CoV-2 virus N protein in 200 μl of Freund's complete adjuvant (Sigma-Aldrich). Subsequently, the mice were boosted by alternating intraperitoneal / subcutaneous injections of a 1:1 emulsion containing 25 μg of SARS-CoV-2 virus N protein in Freund's incomplete adjuvant (Sigma-Aldrich) up to 3 times every two weeks. 4 days prior to myeloma fusion, which exhibited the highest antibody titers (see figure 1 , using serum ELISA method to determine antibody titer) #1 mice were boosted intraperitoneally with 25 μg of SARS-CoV-2 virus N protein (without adjuvant).
[0107] SARS-CoV-2 virus N protein sequence (SEQ ID NO: 1):
[0108] MSDNGPQNQRNA...
Embodiment 2
[0114] Example 2: Variable region sequencing of monoclonal antibodies and antibody recombinant production
[0115]1) Use the rapid ELISA mouse antibody subtype identification kit (Clonotyping System-HRP SouthernBiotech) to identify the subtype of the antibody in the hybridoma cell culture supernatant, and the identification results show that the heavy chain is IgG1, and the light chain is Kappa type; TRIzol (Ambion) from 3×10 6 ~5×10 6 Total RNA was extracted from hybridoma cells and reverse transcribed into cDNA using antibody subtype-specific primers and universal primers (PrimeScriptTM 1stStrand cDNA Synthesis Kit, Takara).
[0116] 2) Subsequent amplification of mouse immunoglobulin heavy chain and light chain V-region fragments by RACE PCR (Nanjing KingScript Biotechnology Co., Ltd.), and subcloning the resulting PCR fragments into the pMD18-T vector system (Takara), The insert is sequenced using vector-specific primers.
[0117] 3) The unique V region amino acid seque...
Embodiment 3
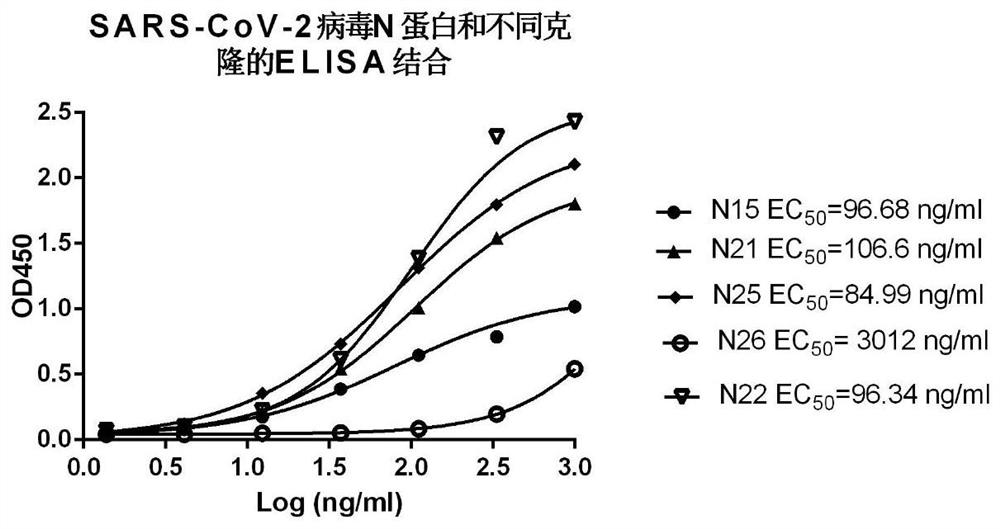
[0154] Example 3: Binding of monoclonal antibodies to recombinant SARS-CoV-2 virus N protein
[0155] 1) Dilute the recombinant SARS-CoV-2 virus N protein to 0.5 μg / ml with PBS buffer.
[0156] 2) Add 100 μl of diluted SARS-CoV-2 virus N protein solution to each well of the ELISA plate (Nunc), and coat and react overnight at 4°C.
[0157] 3) Wash the plate once with PBS-T (0.05% Tween), discard the supernatant. Add 200 μl of blocking solution (100 ml of PBST; 1 g of bovine serum albumin) to each well, and incubate at 37° C. for 0.5 hour.
[0158] 4) Discard the blocking solution, add 100 μl of 10 μg / ml purified antibody to the first well, and dilute with PBS buffer according to a 3-fold gradient, a total of 7 test concentration gradients.
[0159]5) Incubate at room temperature for 1 hour. Wash the plate three times with PBST and discard the supernatant.
[0160] 6) Add 100 μl of horseradish peroxidase-labeled goat anti-human IgG (Nanjing GenScript Biotechnology Co., Ltd.)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com