Application of methionine enzyme gene therapy in treatment of malignant tumors
A gene and suicide gene technology, applied in the field of tumor therapy and gene therapy, can solve side effects and other problems, and achieve long-term expression, gene stability, and large gene fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
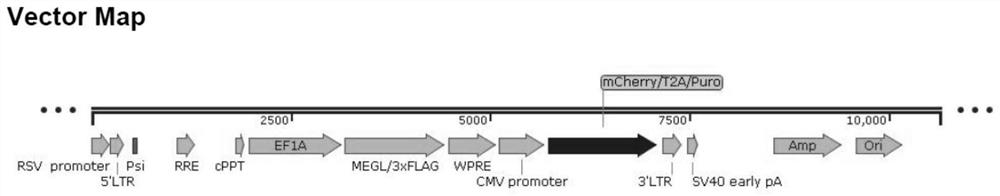
[0036] Example 1: Containing MEGL expression vector construction
[0037] The MEGL / 3xFLAG sequence used is as follows (SEQ ID NO.1):
[0038]
[0039] The viral vector comes from Saiye Biotechnology, and the Gateway cloning technology is mainly used to construct the expression vector. Gateway technology consists of two reactions, BP and LR. The BP reaction uses a recombination reaction between an attB DNA fragment or an expression clone and an attP donor vector to create an entry clone. The LR reaction is a recombination reaction between an attL entry clone and an attR destination vector. details as follows:
[0040] 1) BP reaction construction entry vector (Entry Vector): mix the Gateway expression vector (attB1-MEGL-attB2 sequence) containing the target gene with the donor vector (pDONR) with the attP1-ccdB (suicide gene)-attP2 sequence, Add BP Clonase enzyme mixture containing Int and IHF, incubate at 25°C for 1 hour, and treat with proteinase K at 37°C for 10 minutes. ...
Embodiment 2
[0043] Example 2: Preparation of overexpressed MEGL virus (taking lentivirus as an example)
[0044] 1) Virus packaging: the day before transfection, inoculate 293T cells into a culture dish, and the number of inoculated cells should be 90%-95% confluent on the day of transfection; on the day of transfection, remove the culture medium from the 293T cells , add 10mL (10cm petri dish) culture medium for virus packaging. Prepare calcium phosphate-DNA precipitation as follows: A. Calcium-DNA mixture: first add CaCl to a 5mL sterile EP tube 2 , and then add the auxiliary plasmid and the target gene plasmid respectively and mix well. B. Place the centrifuge tube containing the calcium-DNA mixture on a vortex shaker to vortex the liquid, then add 2×HBS drop by drop, vortex for a few seconds after the drop, and let stand for 5 minutes. Pour the calcium phosphate-DNA suspension into the cell culture medium of the above cells, mix the medium gently, and place it in a 37°C, 5% CO2 satu...
Embodiment 3
[0049] Example 3: Antitumor effect of overexpressing MEGL virus.
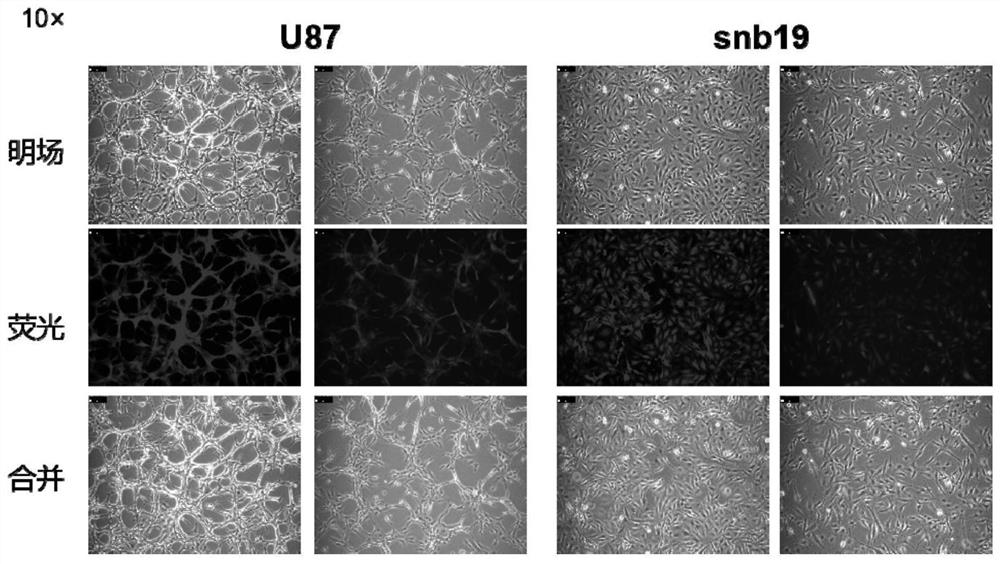
[0050] 1. Take the glioma cells U87 and snb19 in the logarithmic growth phase and inoculate them in a 6-well plate. After the cell density grows to about 30%-50%, add the control empty virus vector according to MOI=10 (hereinafter both defined as V group) and overexpressed MEGL lentivirus (defined as group M below), and added a final concentration of 5 μg / mL Polybrene reagent to aid in staining. After 24 hours, replace it with normal medium for culture, and after continuing to culture for 48 hours, use a final concentration of 2 μg Puromycin / mLl was screened, and the fluorescence intensity and ratio of the cells were observed by a fluorescence microscope every 24 hours. The amount of cells with red fluorescence exceeding 95% indicated that the screening was successful. Cell screening results such as figure 2 shown.
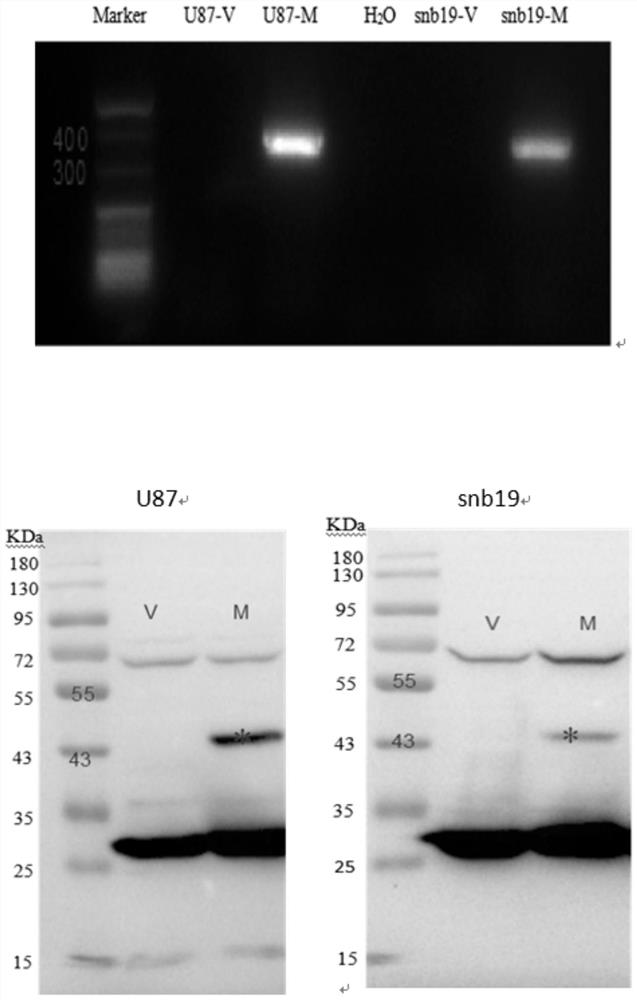
[0051] 2. Using Real time PCR and Western Blot to identify the expression of methionase in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com