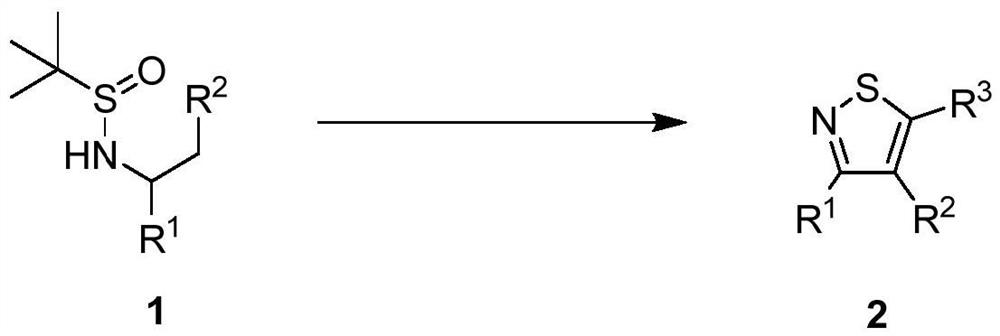
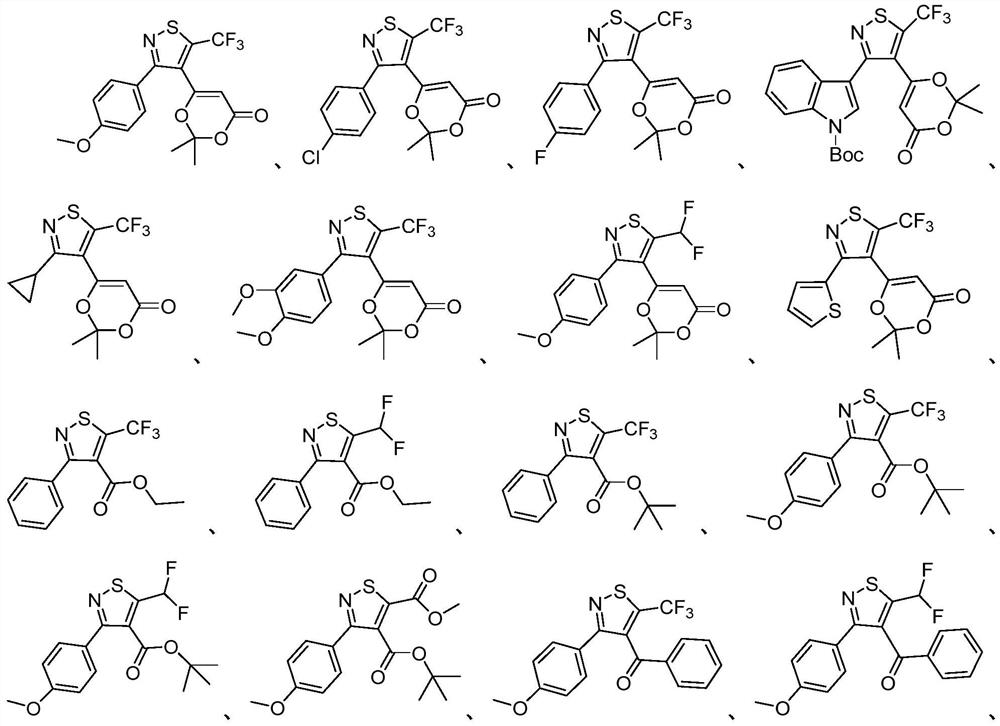
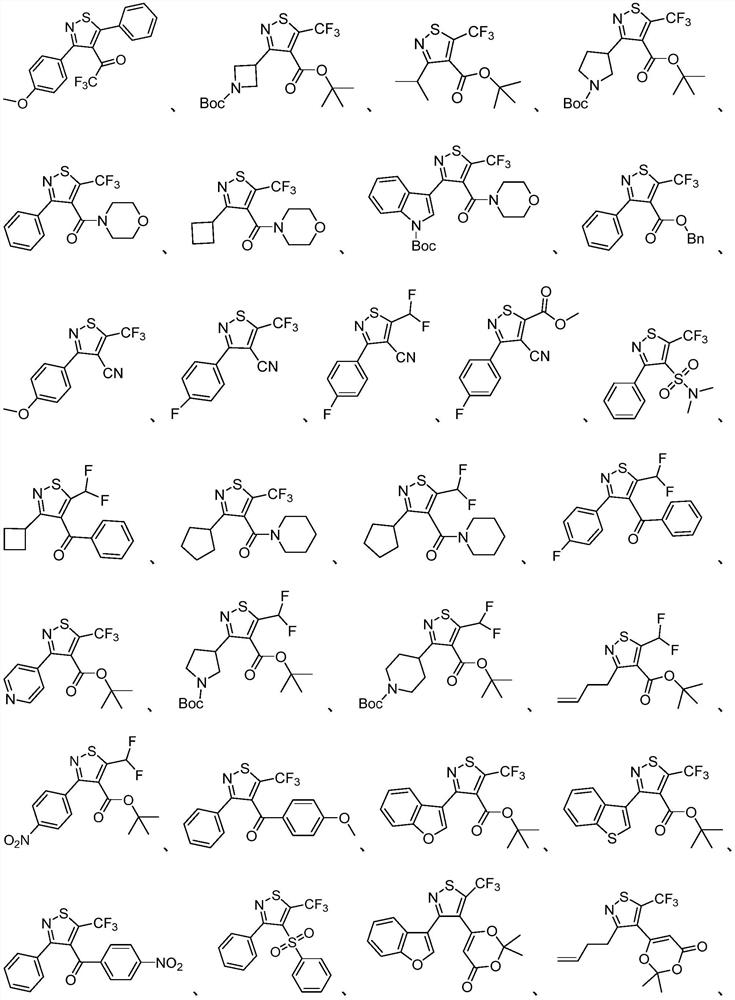
Polysubstituted isothiazole derivative and preparation method thereof
A technology for isothiazole and derivatives, applied in the field of polysubstituted isothiazole derivatives and their preparation, can solve the problems of difficult preparation of raw materials, poor atom economy, etc., and achieve the effects of avoiding heavy metal pollution, easy operation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: The raw material tert-butylsulfinamide derivative compound 1 (381mg, 1mmol) was added in a round bottom flask, and methylene chloride (10mL) was added in the round bottom flask, and after dissolving, DMAP was added at 0°C ( 305mg, 2.5mmol), TFAA (556μL, 4mmol), reacted at 0°C for 10min, then raised the temperature to 25°C for 12h, after the reaction was completed, the solvent was distilled off under reduced pressure, and the product was separated by silica gel column chromatography, and the eluent was petroleum Ether / ethyl acetate (volume ratio 20:1) to obtain polysubstituted isothiazole product 1 (308mg).
[0075]6-(3-(4-methoxyphenyl)-5-(trifluoromethyl)isothiazol-4-yl)-2,2-dimethyl-4H-1,3-dioxin-4-one, yield 80%;
[0076]
[0077] 1 H NMR (400MHz, Chloroform-d) δ7.56 (d, J = 9.0Hz, 2H), 6.95 (d, J = 8.4Hz, 2H), 5.42 (s, 1H), 3.84 (s, 3H), 1.70 (s,6H). 13 C NMR (101MHz, Chloroform-d) δ168.20, 161.27, 160.01, 158.67, 155.97 (q, 2 J C-F =3.6Hz), 129.9...
Embodiment 2
[0078] Example 2: The raw material tert-butylsulfinamide derivative compound 1 (385mg, 1mmol) was added in a round-bottomed flask, and chloroform (10mL) was added to the round-bottomed flask, and after dissolving, DMAP was added at 0°C ( 305mg, 2.5mmol), TFAA (556μL, 4mmol), reacted at 0°C for 12min, then raised the temperature to 50°C for 12h, after the reaction, the solvent was distilled off under reduced pressure, and the product was separated by silica gel column chromatography, and the eluent was petroleum Ether / ethyl acetate (volume ratio 20:1) to obtain multi-substituted isothiazole product 2 (175 mg);
[0079] 6-(3-(4-chlorophenyl)-5-(trifluoromethyl)isothiazol-4-yl)-2,2-dimethyl-4H-1,3-dioxin-4-one, yield 45%;
[0080]
[0081] 1 H NMR (400MHz, Chloroform-d) δ7.57(d, J=8.6Hz, 2H), 7.44(d, J=8.5Hz, 2H), 5.44(s, 1H), 1.70(s, 6H). 13 C NMR (101MHz, Chloroform-d) δ167.03, 159.66, 158.02, 158.67, 156.37 (q, 2 J C-F =3.6Hz), 136.67, 131.84, 129.65, 129.11, 120.69 (q,...
Embodiment 3
[0082] Example 3: The raw material tert-butylsulfinamide derivative compound 1 (369mg, 1mmol) was added to a round bottom flask, and chloroform (10mL) was added to the round bottom flask, and after dissolving, DMAP was added at 0°C ( 305mg, 2.5mmol), TFAA (556μL, 4mmol), reacted at 0°C for 10min, then raised the temperature to 50°C for 12h, after the reaction was completed, the solvent was distilled off under reduced pressure, and the product was separated by silica gel column chromatography, and the eluent was petroleum Ether / ethyl acetate (volume ratio 30:1), to obtain multi-substituted isothiazole product 3 (183mg);
[0083] 6-(3-(4-fluorophenyl)-5-(trifluoromethyl)isothiazol-4-yl)-2,2-dimethyl-4H-1,3-dioxin-4-one, yield 63%;
[0084]
[0085] 1 H NMR (400MHz, Chloroform-d) δ7.63–7.59 (m, 2H), 7.14 (d, J=1.9Hz, 2H), 5.43 (s, 1H), 1.68 (s, 6H). 13 C NMR (101MHz, Chloroform-d) δ167.31, 163.98 (d, 1 J C-F =250.0Hz), 159.77, 158.21, 156.32 (q, 2 J C-F =38.0Hz), 130.50(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com