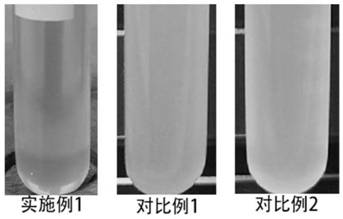
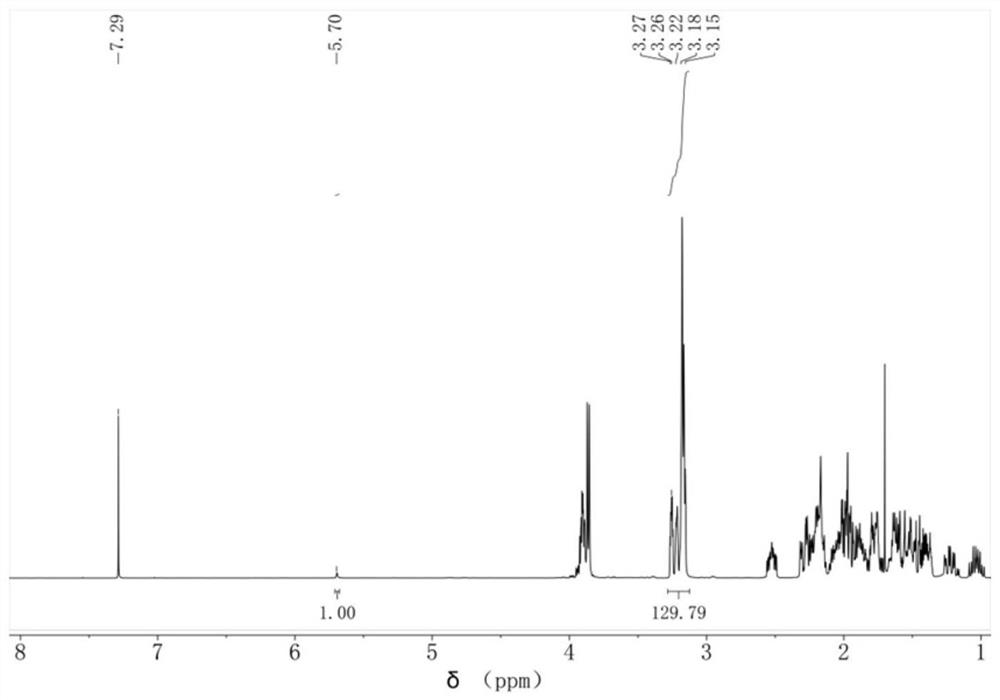
Phase-transfer catalytic synthesis method of 3, 4-epoxy cyclohexyl methyl-3', 4'-epoxy cyclohexyl formate
A technology of phase transfer catalysis and hexylmethyl, which is applied in the field of organic synthesis, can solve the problems of complex catalyst preparation process, unavailable raw materials, and low catalytic yield, and achieve the effect of large market application value, low cost, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) In the reaction vessel, add 20g of dichloromethane and 0.2g of sodium ethoxide catalyst, under stirring, rise to 35°C, add 100g of 3-cyclohexene-1-carbaldehyde dropwise through the constant pressure funnel, and react at constant temperature for 1h after dropping. After washing with 3.7% dilute hydrochloric acid, separating liquid and distilling under reduced pressure, the epoxidized intermediate 3,4-cyclohexenecarboxylate-3',4'-cyclohexene methyl ester was obtained.
[0042] (2) 50g 3,4-cyclohexenecarboxylic acid-3',4'-cyclohexene methyl ester, 100g dichloroethane, 2.8g phase transfer catalyst [C 16 h 33 N(CH 3 ) 3 ] 3 PW 4 o 24 , 0.16g recovering agent cetyltrimethylammonium bromide, 0.4g buffering agent sodium bicarbonate were added to the reaction vessel, the temperature was raised to 55°C and refluxed, 75g 30% hydrogen peroxide was added dropwise at constant pressure and reacted at constant temperature for 1 hour, and the reaction was completed Cool to roo...
Embodiment 2
[0048] (1) In the reaction vessel, add 15g of dichloroethane and 0.5g of aluminum isopropoxide catalyst, under stirring, rise to 37°C, add 100g of 3-cyclohexene-1-carbaldehyde dropwise into the constant pressure funnel, and keep the temperature constant After reacting for 1 h, washing with 3.7% dilute hydrochloric acid, separating liquid and distilling under reduced pressure, the epoxidized intermediate 3,4-cyclohexenecarboxylate-3',4'-cyclohexene methyl ester was obtained.
[0049] (2) 50g 3,4-cyclohexenecarboxylic acid-3', 4'-cyclohexene methyl ester, 100g dichloroethane, 3g phase transfer catalyst [C 18 h 37 N(CH 3 ) 3 ] 3 PW 4 o 24 , 0.18g recovering agent octadecyltrimethylammonium bromide, 0.5g buffering agent dipotassium hydrogen phosphate were added to the reaction vessel, the temperature was raised to 60°C and refluxed, 75g 30% hydrogen peroxide was added dropwise at constant pressure for 1 hour at constant temperature, and the reaction After cooling to room tem...
Embodiment 3
[0053] (1) In the reaction vessel, add 18g of chloroform and 0.15g of sodium methoxide catalyst, under stirring, rise to 40°C, add 100g of 3-cyclohexene-1-carbaldehyde dropwise into the constant pressure funnel, and react at constant temperature for 1h after dropping. After washing with 3.7% dilute hydrochloric acid, separating liquid and distilling under reduced pressure, the epoxidized intermediate 3,4-cyclohexenecarboxylate-3',4'-cyclohexene methyl ester was obtained.
[0054] (2) 50g 3,4-cyclohexenecarboxylic acid-3', 4'-cyclohexene methyl ester, 100g chloroform, 2.8g phase transfer catalyst [C 16 h 33 N(CH 3 ) 3 ] 3 PW 4 o 24 , 0.16g recovery agent cetyltrimethylammonium bromide, 0.35g buffering agent sodium acetate were added to the reaction vessel, the temperature was raised to 65°C and refluxed, 75g 30% hydrogen peroxide was added dropwise at constant pressure to complete the constant temperature reaction for 1h, and the reaction was completed and cooled to room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com