Construction of immune-activated recombinant lactococcus lactis and application of immune-activated recombinant lactococcus lactis as tumor vaccines, immunologic adjuvants and like
A Lactococcus lactis, immune activation technology, applied in the fields of biomedicine and bioengineering, can solve the problem that immune active cells cannot fully exert specific immune cytotoxic effects, etc., to improve the tumor microenvironment, reduce immunosuppressive activity, and promote survival. and the effect of amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Construction of Lactococcus lactis pNZ8148-Flt3L-OX40L:
[0034] According to SEQ ID NO.1, design and optimize the gene sequence of its coding gene as shown in SEQ ID NO.2, and design the corresponding restriction site.
[0035] The pNZ8148 vector plasmid was extracted, and the pNZ8148 vector plasmid and the target gene were digested with NcoI / HindIII; 37°C, 2h; then electrophoresed on 1% agarose gel, and the electrophoresis results were observed. The DNA recovery kit was used to recover the target gene fragment and the pNZ8148 vector plasmid double digestion product. The linearized fragment of the recovered pNZ8148 vector plasmid and the target gene fragment are ligated into a closed circular DNA molecule under the action of T4 DNA ligase through complementary cohesive ends to form the recombinant plasmid pNZ8148-Flt3L-OX40L.
[0036] The ligated recombinant plasmid was sent to be sequenced. The sequencing results were accurate and there were no frameshift mutations. ...
Embodiment 2
[0038] Construction of Lactococcus lactis pNZ8148-Flt3L-OX40L-cACMA:
[0039] According to SEQ ID NO.3, design and optimize the gene sequence of its coding gene as shown in SEQ ID NO.4, and design the corresponding enzyme cutting sites.
[0040] The pNZ8148 vector plasmid was extracted, and the pNZ8148 vector plasmid and the target gene were digested with NcoI / HindIII; 37°C, 2h; then electrophoresed on 1% agarose gel, and the electrophoresis results were observed. The DNA recovery kit was used to recover the target gene fragment and the pNZ8148 vector plasmid double digestion product. The linearized fragment of the recovered pNZ8148 vector plasmid and the target gene fragment are ligated into a closed circular DNA molecule through complementary cohesive ends under the action of T4 DNA ligase to form the recombinant plasmid pNZ8148-Flt3L-OX40L-cACMA.
[0041] The ligated recombinant plasmid was sent to be sequenced. The sequencing results were accurate and there were no frameshi...
Embodiment 3
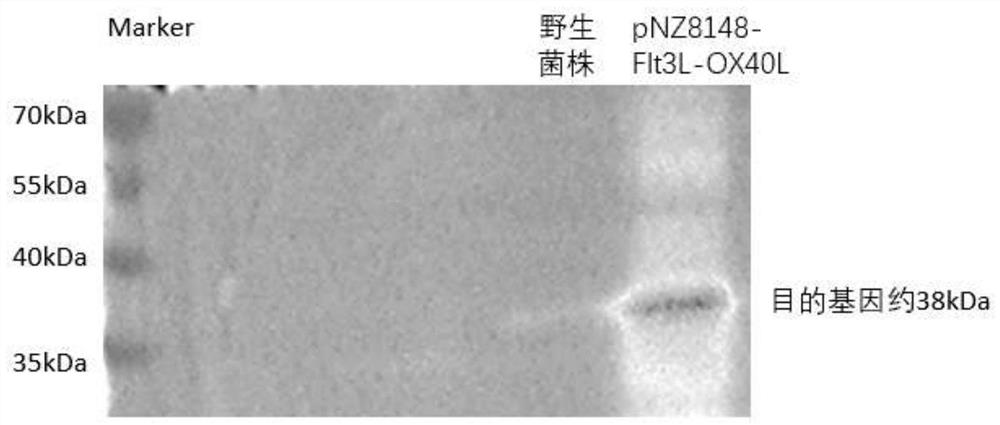
[0043] Expression and detection of recombinant Lactococcus lactis pNZ8148-Flt3L-OX40L:
[0044] The recombinant plasmid pNZ8148-Flt3L-OX40L was transformed into lactic acid bacteria for protein expression and detection.
[0045] (1) Induced expression of pNZ8148-Flt3L-OX40L: The verified correct recombinant expression plasmid pNZ8148-Flt3L-OX40L was electrotransformed into Lactococcus NZ9000 to obtain recombinant lactic acid bacteria pNZ8148-Flt3L-OX40L, and the positive clone (pNZ8148-Flt3L- OX40L) was inoculated in a test tube of 5mL M17+0.5%glucose+10μg / mL chloramphenicol culture solution, cultured overnight at 30°C; the next day was inoculated in M17+0.5%glucose+10μg / mL chloramphenicol culture solution at a ratio of 1:100, Cultivate statically at 30°C until the OD600 of the bacteria is about 0.3-0.5; add Nisin to a final concentration of 10ng / mL, and culture at 30°C for 3-4 hours to induce the expression of the fusion protein.
[0046] (2) Detection of the expression of p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com