Analysis method for extracting immune polypeptide in biological sample based on immunoprecipitation method
A technology of immunoprecipitation and immune peptides, which is applied in the field of analysis of immune peptides in biological samples based on immunoprecipitation, can solve problems such as polymer contamination, enrichment and peptide loss, and achieve high specificity and effectiveness. The effect of high application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
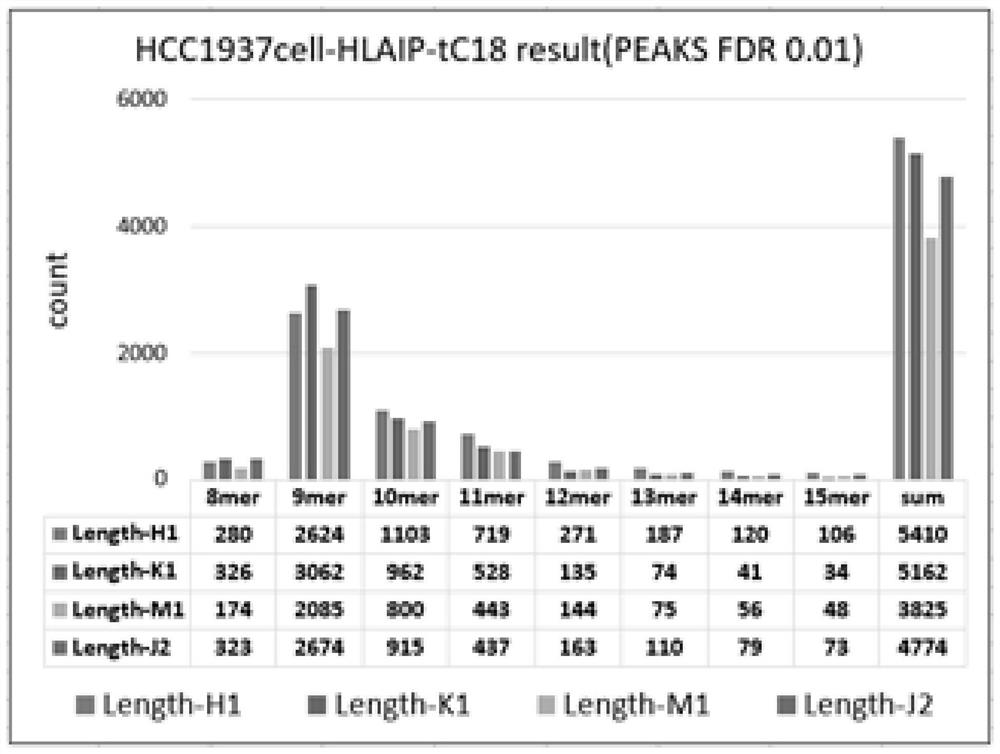
[0029] Example 1: Extraction and mass spectrometric identification of immune polypeptides from HCC1937 cells.
[0030] The reagents used in this example were purchased from Sigma unless otherwise noted, including triethanolamine, DMP cross-linking agent, Tris, acetonitrile, trifluoroacetic acid, and acetic acid. Protein A sepharose 4B, protease inhibitors, pepstatinprotease inhibitors and PBS were purchased from Thermo Fisher Scientific. The desalting column SepPak C18 Cartridges was purchased from Waters. Econo-column empty columns were purchased from Bio-Rad.
[0031] An analysis method for extracting immune polypeptides in biological samples based on immunoprecipitation, which first coats the specific antibody (W6 / 32 antibody) corresponding to the target immune polypeptide to magnetic beads through cross-linking reagents, and then enriches through magnetic bead precipitation The target immune polypeptide (HLA-peptide in HCC1937 cell lysate), the enriched target immune pol...
Embodiment 2
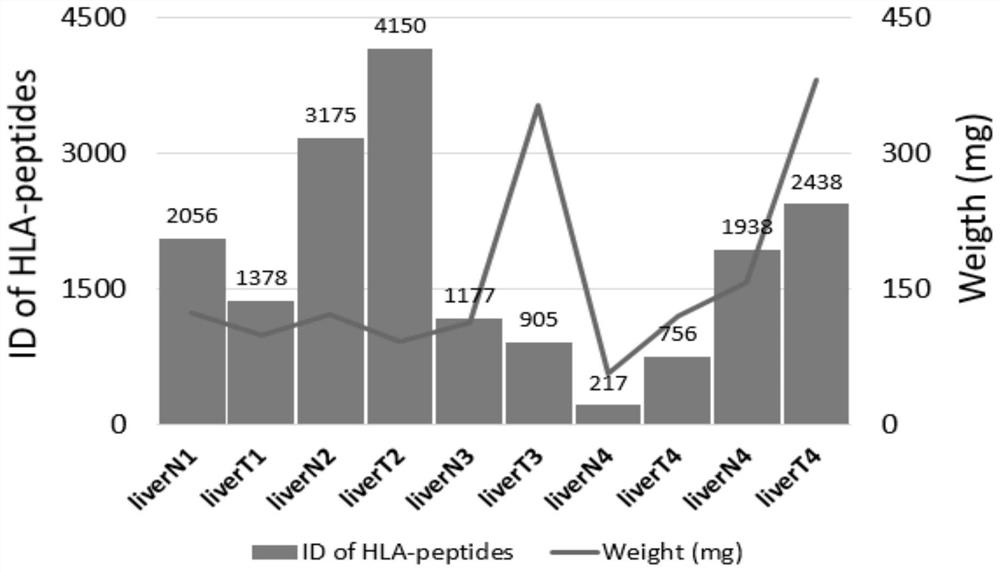
[0045] Example 2: Identification results of immunological peptides in tissues of 5 patients and distribution diagrams of peptide lengths.
[0046] The method used in the second embodiment is the same as that in the first embodiment, only the cell line samples are replaced with patient tissue samples.
[0047] 5 patient tissue samples (liver cancer tissue) were taken for extraction of immune polypeptides. In addition to the difference between the tissue lysis method and the cell line, the preparation of the immunoaffinity column, the immunoaffinity binding, elution, purification and desalting of the lysate, the detection and data analysis of mass spectrometry are all consistent with Example 1, and the tissue lysis method details as follows:
[0048]Each tissue needs more than 200mg, add about 20mL of cell lysate, then homogenize with tissue magnetic beads, and incubate on ice for 1 hour, and the solution can be pipet or inverted every 10 minutes. Then, put the lysed tissue sa...
Embodiment 3
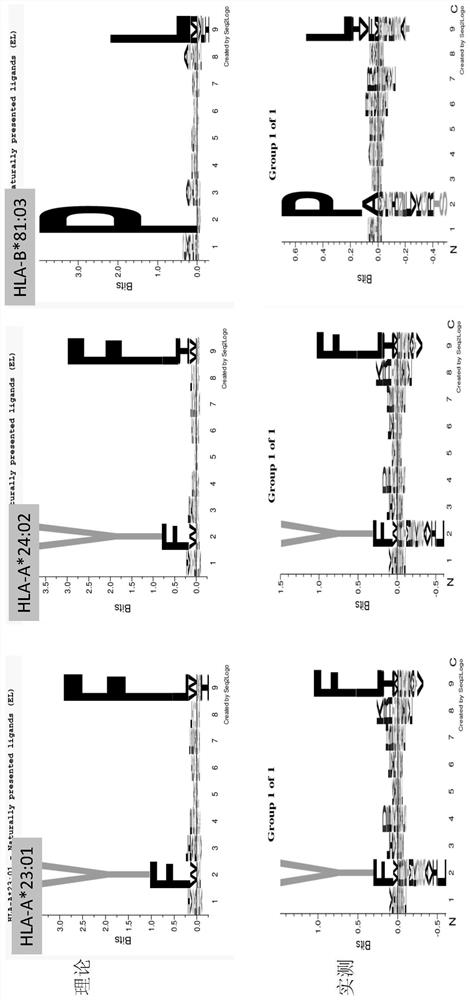
[0050] Example 3: Verification of the enriched HLA in Example 1 and Example 2.
[0051] The acrylamide electrophoresis gel 10% NuPAGE precast gel, 10× reducing agent, 4× loading solution, 20× electrophoresis buffer, western blot imprinting membrane and filter paper used in this example were all purchased from Thermo Company; the molecular weight of prestained protein The marker was purchased from Bio-rad Company; the primary anti-beta 2-microglobulin rabbit recombinant antibody (anti-beta 2microglobulin) was purchased from Abcam Company; the secondary antibody horseradish peroxidase-labeled goat anti-rabbit IgG was purchased from Beyontian Company; electrophoresis The instrument MiniGel Tank and iBlot2 dry transfer system were purchased from Thermo Company.
[0052] Take one part of each of the effluents 2 in Examples 1 and 2, dissolve them in PBS, measure the protein concentration with OD280, and take 200ng for SDS-PAGE running gel and WB analysis to verify the success of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com