Method for separating lignin-based p-hydroxybenzaldehyde, vanillin and syringaldehyde
A technology based on p-hydroxybenzaldehyde and lignin, which is applied in the fields of biomass energy and chemical industry, can solve the problems of being volatile and unsuitable for large-scale production, and achieves the effect of low price, suitable for large-scale production, and green and environmental protection in the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
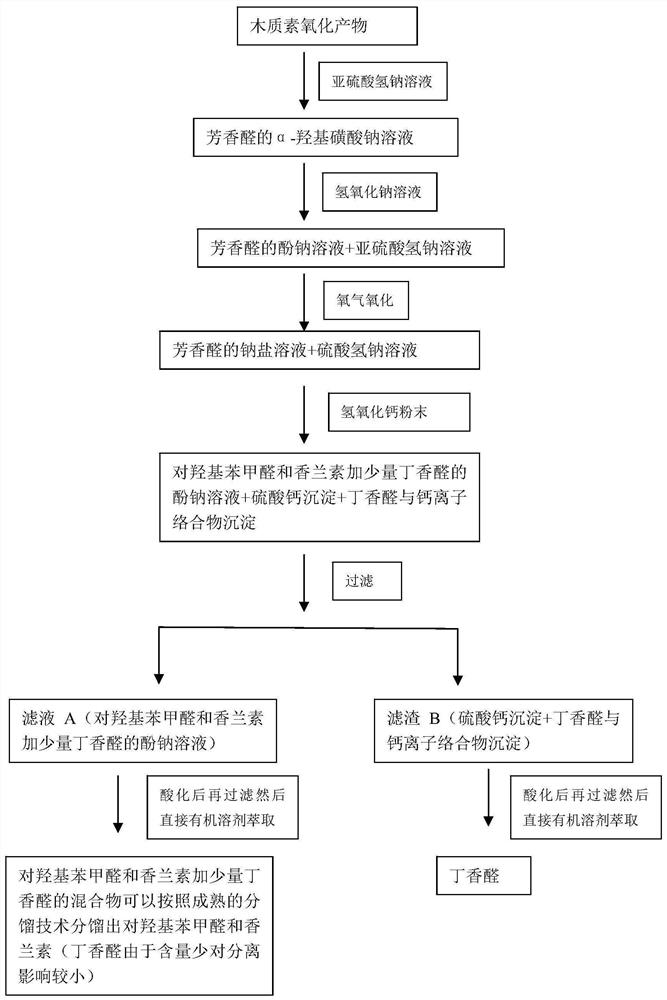
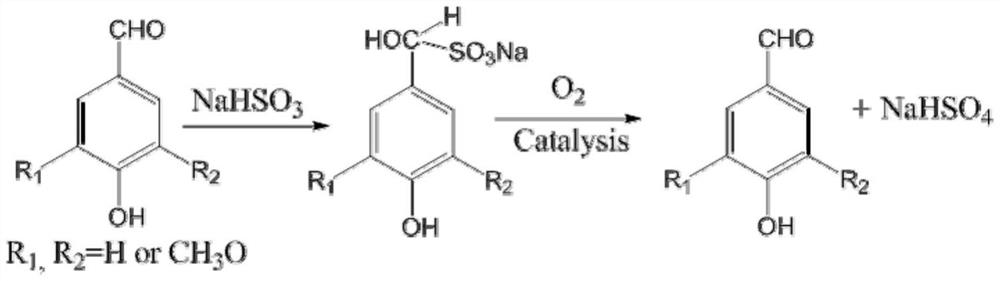
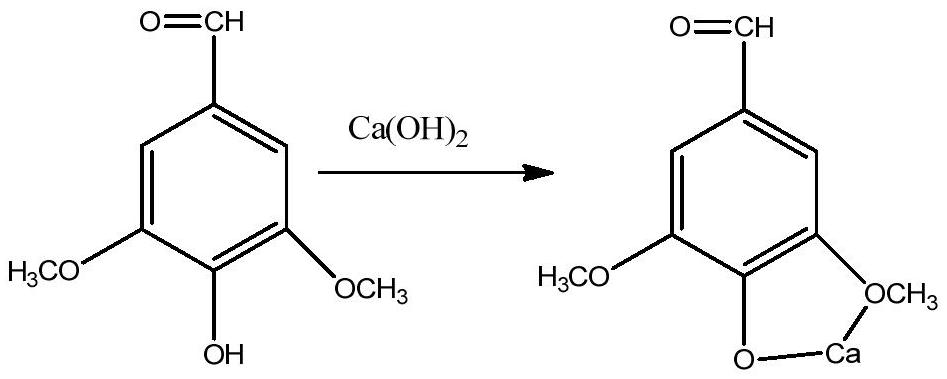
[0029] 10 grams of lignin oxygen oxidation products, tested by liquid chromatography, contained 1.1 grams of p-hydroxybenzaldehyde, 2.3 grams of vanillin, and 3.2 grams of syringaldehyde in the oxidation products, added 300 mL of distilled water, 11 grams of sodium bisulfite, and stirred at room temperature for 4 hours , then filter, get the filtrate and join in the reactor of 500mL, add sodium hydroxide (analytical pure) 5.0 grams in the reactor, after stirring for 10 minutes, then add 0.1 gram of anhydrous copper sulfate (analytical pure) in the reactor, Close the reactor, feed 2Mpa oxygen, react at 50°C for 30 minutes, open the reactor after the reaction, transfer all the reaction solution to a 500mL volumetric flask and dilute it to 500mL with water. Transfer 1mL from the 500mL volumetric flask to a 10mL volumetric flask and dilute it to 10mL with water. Get a small amount of liquid from the 10mL volumetric flask and do ion chromatography to detect and find that all sulfat...
Embodiment 2
[0034]10 grams of lignin oxygen oxidation products, tested by liquid chromatography, contained 1.1 grams of p-hydroxyphenylacetaldehyde, 2.3 grams of vanillin, and 3.2 grams of syringaldehyde in the oxidation products, added 300 mL of distilled water, 11 grams of sodium bisulfite, and stirred at room temperature for 4 hour, then filter, get the filtrate and join in the reactor of 500mL, then add sodium hydroxide (analytical pure) 5.0 grams, after stirring for 10 minutes, then add 0.1 gram of anhydrous cobalt acetate (analytical pure) in the reactor, close The reaction kettle was fed with 3Mpa oxygen, and reacted at 35°C for 70 minutes. After the reaction was completed, the reaction kettle was opened, and all the reaction liquid was transferred to a 500mL volumetric flask to make the volume to 500mL with water. Transfer 1mL from the 500mL volumetric flask to a 10mL volumetric flask and dilute it to 10mL with water. Get a small amount of liquid from the 10mL volumetric flask and...
Embodiment 3
[0039] 10 grams of lignin oxygen oxidation products, tested by liquid chromatography, contained 1.1 grams of p-hydroxyphenylacetaldehyde, 2.3 grams of vanillin, and 3.2 grams of syringaldehyde in the oxidation products, added 300 mL of distilled water, 11 grams of sodium bisulfite, and stirred at room temperature for 4 hour, then filter, get the filtrate and join in the reactor of 500mL, add sodium hydroxide (analytical pure) 5.0 grams in the reactor, after stirring for 10 minutes, then add 0.1 gram of anhydrous manganese acetate (analytical pure) in the reactor , close the reactor, feed 4Mpa oxygen, react at 60°C for 20 minutes, open the reactor after the reaction, transfer all the reaction solution to a 500mL volumetric flask and dilute it to 500mL with water. Transfer 1mL from the 500mL volumetric flask to a 10mL volumetric flask and dilute it to 10mL with water. Get a small amount of liquid from the 10mL volumetric flask and do ion chromatography detection and find that al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com