Preparation method and application of HA material for HA soluble microneedle
A soluble, microneedle technology, applied in the directions of microneedles, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Needs, the hardness of the soluble drug-loaded microneedle tip is not up to standard, and the drying cycle of the finished product is long, so as to achieve the effects of increasing the drug-loading capacity and formability, high drug-loading capacity and drug delivery efficiency, and shortening the drying time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
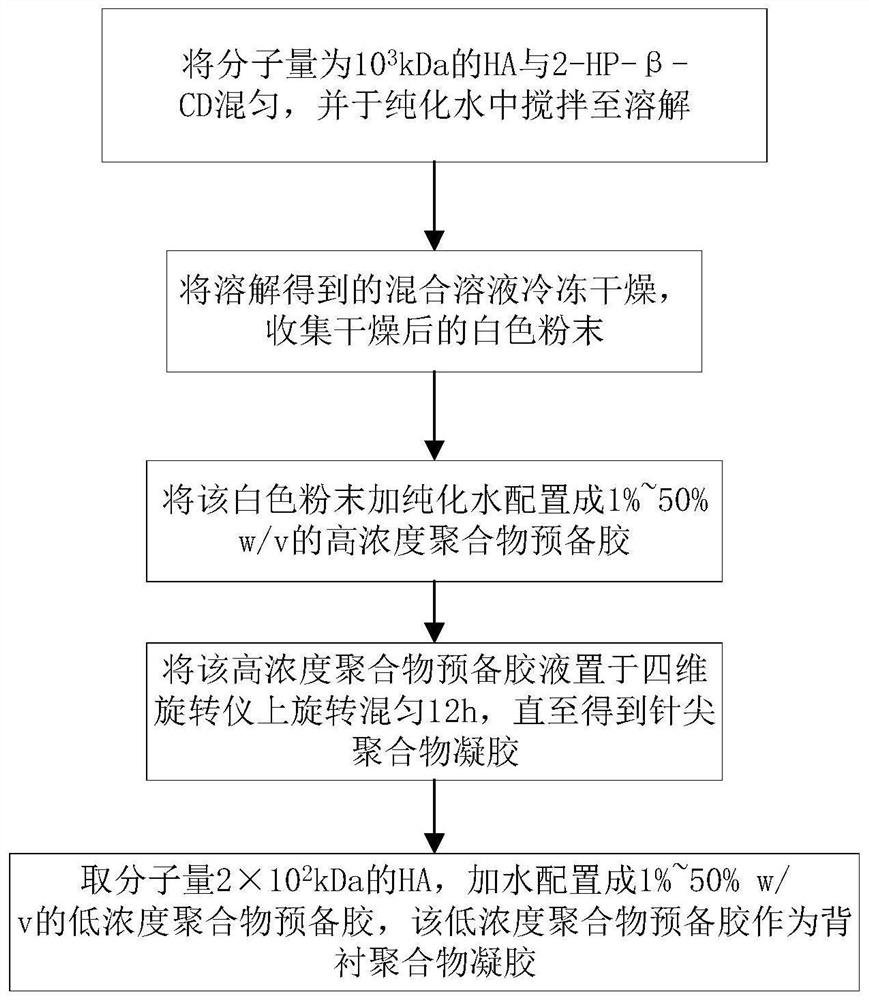
[0037] like figure 1 As shown, the HA material preparation method for HA soluble microneedles comprises the following steps:
[0038] S100, set the molecular weight to 10 3 kDa HA mixed with 2-hydroxymethyl-β-cyclodextrin, and stirred in purified water until dissolved; wherein, the mass ratio of HA to 2-hydroxymethyl-β-cyclodextrin is 1 to 100:1 ~100.
[0039] In fact, in this step, in order to improve the poor formability and weak drug loading capacity of pure HA materials, the molecular weight of 10 3 kDa HA and 2-hydroxymethyl-β-cyclodextrin (2-HP-β-CD) were mixed at a mass ratio of 1:5, and stirred in 10 mL of purified water until dissolved, since HA and 2-HP-β -CD is mixed and dissolved in a certain proportion, so that the hydroxyl group of HA in the system and the carboxyl group of cyclodextrin form a relatively stable and firm hydrogen bond, which greatly increases the stability, hardness and toughness of HA, and after the hydrogen bond is formed between the carboxyl...
Embodiment 2
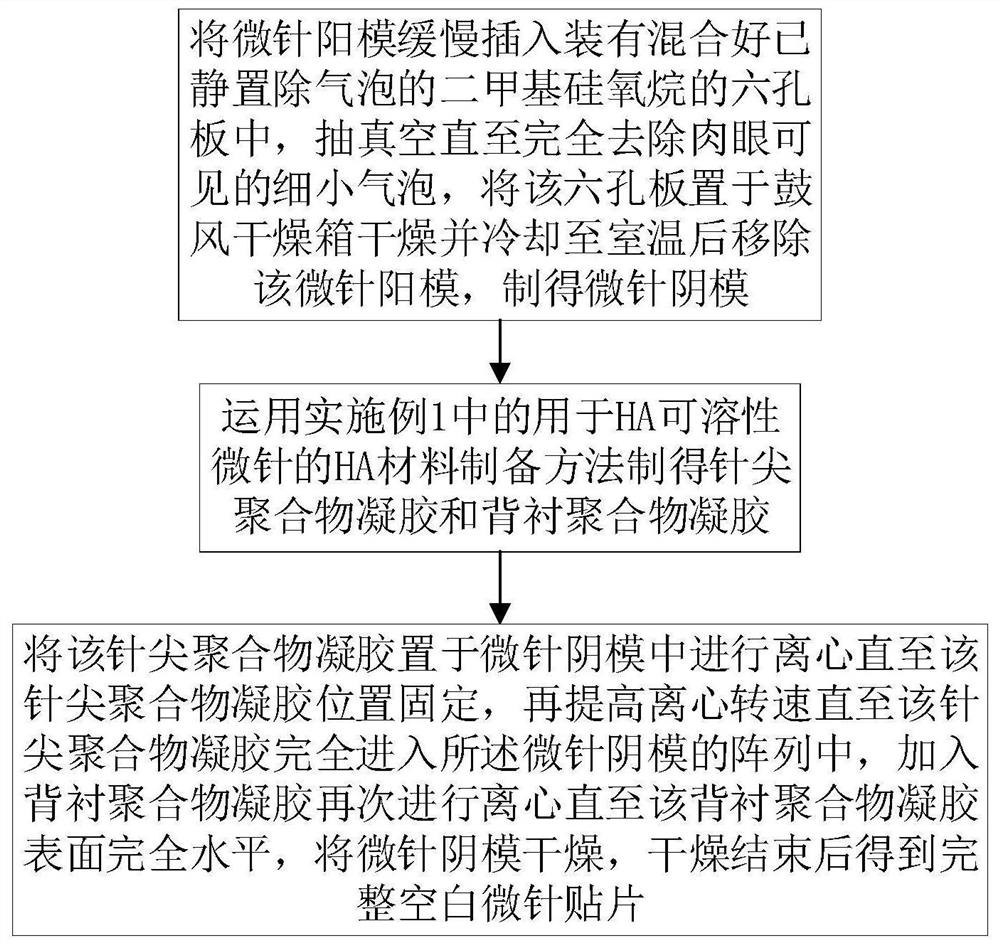
[0048] like figure 2 As shown, in this example, the tip polymer gel and the backing polymer gel obtained in Example 1 are used to prepare a complete blank microneedle patch, including the following steps:
[0049] S000. Slowly insert the male mold of the microneedle into a six-hole plate filled with dimethylsiloxane (PDMS) that has been left to remove air bubbles, and vacuumize for 3 hours to completely remove the tiny air bubbles visible to the naked eye. The plate was dried in a blast drying oven at 70°C for 2 hours, and after cooling to room temperature, the male microneedle mold was removed to obtain the negative microneedle mold;
[0050] The number of microneedle male mold arrays in this step is 15×15 conical metal microneedles (the base diameter of a single needle is 130 μm, the tip diameter is 12 μm, and the needle length is 800 μm), the purpose is to increase the number of microneedle arrays per unit area , and this microneedle male mold can be used precisely becaus...
Embodiment 3
[0055] like image 3 As shown, the preparation of drug-loaded microneedles based on Example 1 and Example 2, that is, the preparation of a complete drug-containing microneedle patch, includes the following steps:
[0056] S000. Slowly insert the male mold of the microneedle into a six-hole plate filled with dimethylsiloxane (PDMS) that has been left to remove air bubbles, and vacuumize for 3 hours to completely remove the tiny air bubbles visible to the naked eye. The plate was dried in a blast drying oven at 70°C for 2 hours, and after cooling to room temperature, the male microneedle mold was removed to obtain the negative microneedle mold;
[0057] S100, using the HA material preparation method for HA soluble microneedles in Example 1 to prepare backing polymer gel and white powder;
[0058] S201. Take the remaining part of the white powder in step S100, mix the indocyanine green model drug with the white powder at a ratio of 2:1 v / w, add purified water to prepare a 15% m / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Tip diameter | aaaaa | aaaaa |
| Needle length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com