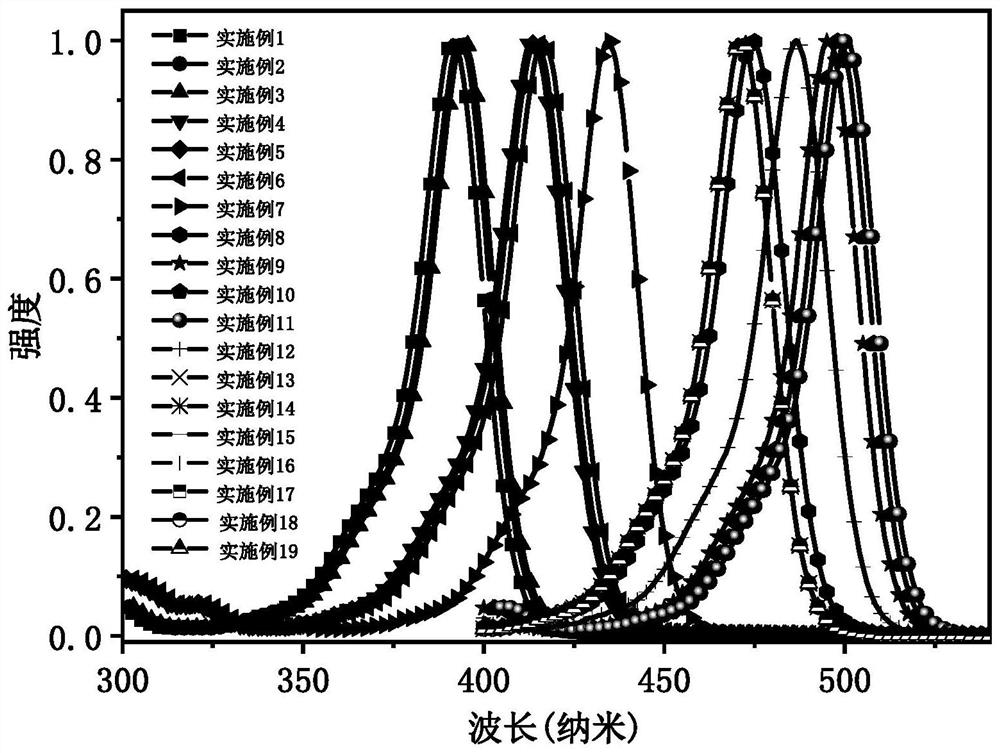
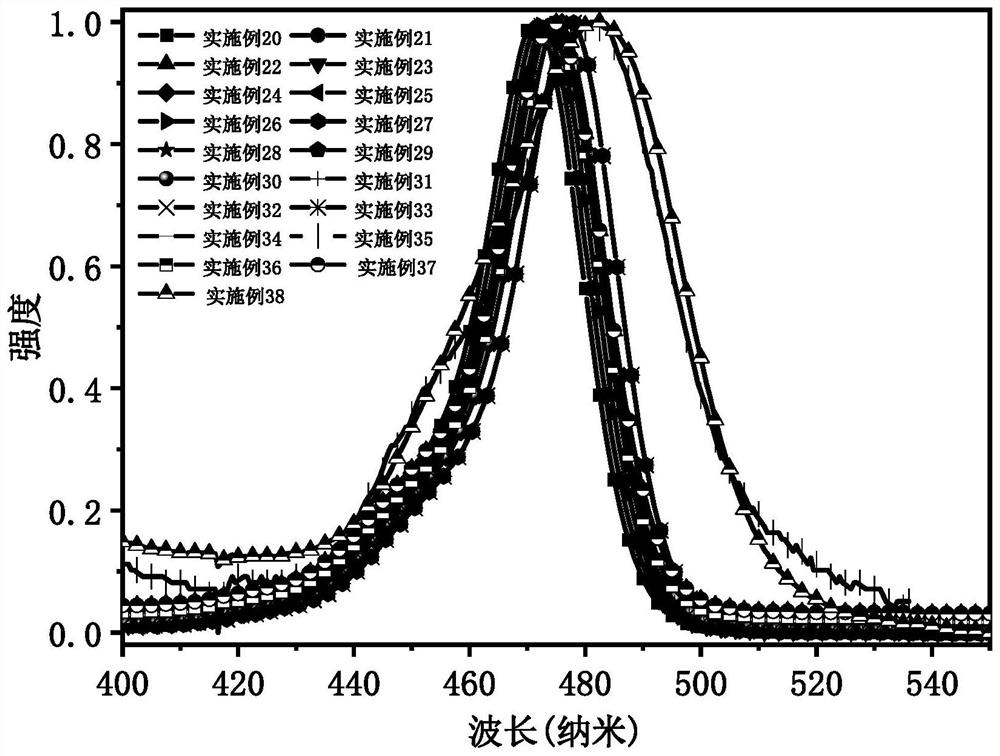
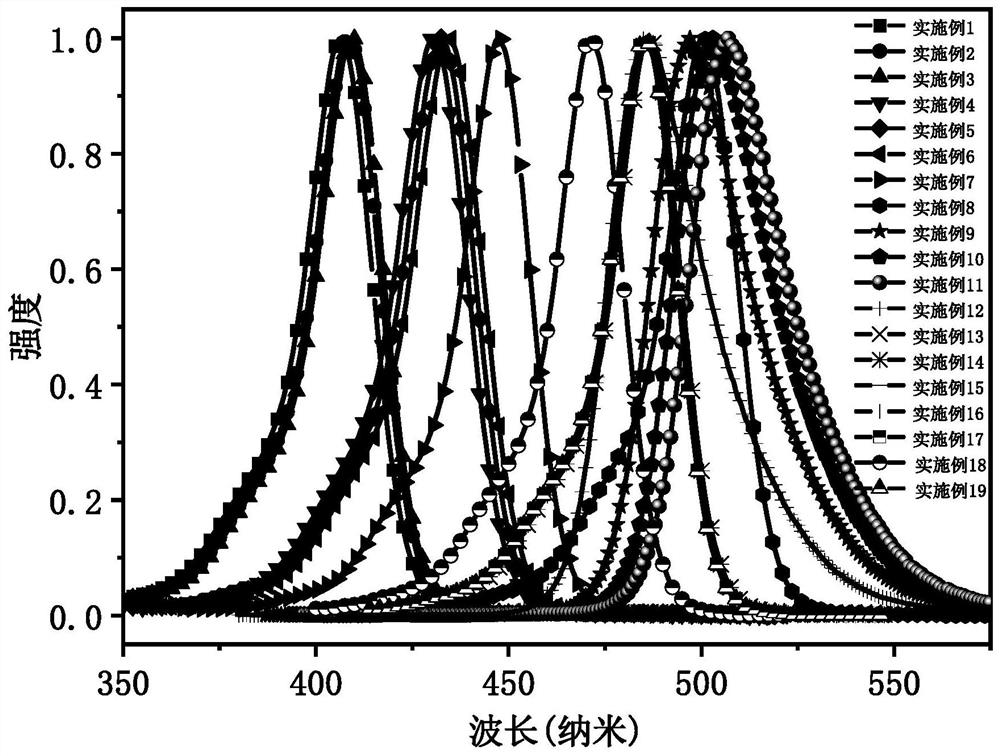
Narrow-emission efficient multi-resonance light-emitting material, preparation method thereof and organic light-emitting diode
A luminescent material and high-efficiency technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as emission peak broadening and spectral red shift, and achieve high color purity, narrow emission spectrum, and excellent photochemical stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This example provides a narrow-emission high-efficiency multiple resonance luminescent material, which is denoted as compound 1. The synthetic route of compound 1 is as follows:
[0084]
[0085] The synthetic method of compound 1 specifically comprises the following steps:
[0086] Add Cz-Br (4.87g, 10mmol), O-Bpin (3.96g, 10mmol), K 2 CO 3 (2.76g, 20mmol), tetrabutylammonium bromide (Bu 4 NBr, 322mg, 1mmol) and Pd(PP 3 ) 4 (578 mg, 0.5 mmol) and degassed. After adding redistilled THF and distilled water by syringe, N 2 Reflux at 80°C for 24h under atmosphere. After the reaction was completed, when the temperature dropped to room temperature, the liquid was separated, the aqueous phase was extracted three times with dichloromethane, and the organic phase was combined, dried with magnesium sulfate, filtered, and the filtrate was concentrated by a rotary evaporator, and separated by column chromatography to obtain compound 1. White solid, 47% yield. 1 H NMR (C...
Embodiment 2
[0088] This example provides a narrow-emission high-efficiency multiple resonance luminescent material, which is denoted as compound 2. The synthetic route of compound 2 is as follows:
[0089]
[0090] The synthetic method of compound 2 specifically comprises the following steps:
[0091] The specific steps are the same as those of compound 1 in Example 1, except that O-Bpin (3.96 g, 10 mmol) in Example 1 is replaced by S-Bpin (4.28 g, 10 mmol).
[0092] Compound 2 was obtained as a white solid with a yield of 49%. 1 H NMR (CDCl 3 ,500MHz)δ(ppm):8.32(2H,dd,J 1 =7.5Hz,J 2 =1.5Hz),7.91-7.90(3H,m),7.46(2H,ddd,J 1 =8.5Hz,J 2 =7.5Hz,J 2 =1.5Hz),7.44(4H,d,J=8.0Hz),7.30(2H,dd,J 1 =8.5Hz,J 2 =7.5Hz),7.28(4H,t,J=7.5Hz),7.10-7.20(4H,m),7.00(4H,t,J=7.5Hz),6.90(2H,dd,J 1 =8.0Hz,J 2 = 2.0 Hz), 6.80 (2H, d, J = 9.0 Hz). Mass (MALDITOF): m / z, calcd for C 48 h 29 BN 2 S 2 + :708.18652; found: 708.18666.
Embodiment 3
[0094] This example provides a narrow-emission high-efficiency multiple resonance luminescent material, which is denoted as compound 3. The synthetic route of compound 3 is as follows:
[0095]
[0096] The synthetic method of compound 3 specifically comprises the following steps:
[0097] The specific steps are the same as those of compound 1 in Example 1, except that O-Bpin (3.96 g, 10 mmol) in Example 1 is replaced by Se-Bpin (5.22 g, 10 mmol).
[0098] Compound 3 was obtained as a white solid with a yield of 35%. 1 H NMR (CDCl 3 ,500MHz)δ(ppm):8.30(2H,dd,J 1 =7.5Hz,J 2 =1.5Hz),7.91-7.90(3H,m),7.40(2H,ddd,J 1 =8.5Hz,J 2 =7.5Hz,J 2 =1.5Hz),7.44(4H,d,J=8.0Hz),7.30(2H,m),7.28(4H,m),7.10-7.20(4H,m),7.00(4H,t,J=7.5Hz ),6.90(2H,dd,J 1 =8.0Hz,J 2 = 2.0 Hz), 6.80 (2H, d, J = 9.0 Hz). Mass (MALDITOF): m / z, calcd for C 48 h 29 BN 2 Se 2 + :804.07542; found: 804.07533.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com