Polypeptide and application and pharmaceutical composition thereof
A composition and drug technology, applied in the field of biomedicine, can solve the problems affecting the quality of life of children, unclear pathogenesis, and high mortality, and achieve the effects of restoring intestinal tissue shape, structure integrity, and reducing intestinal damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
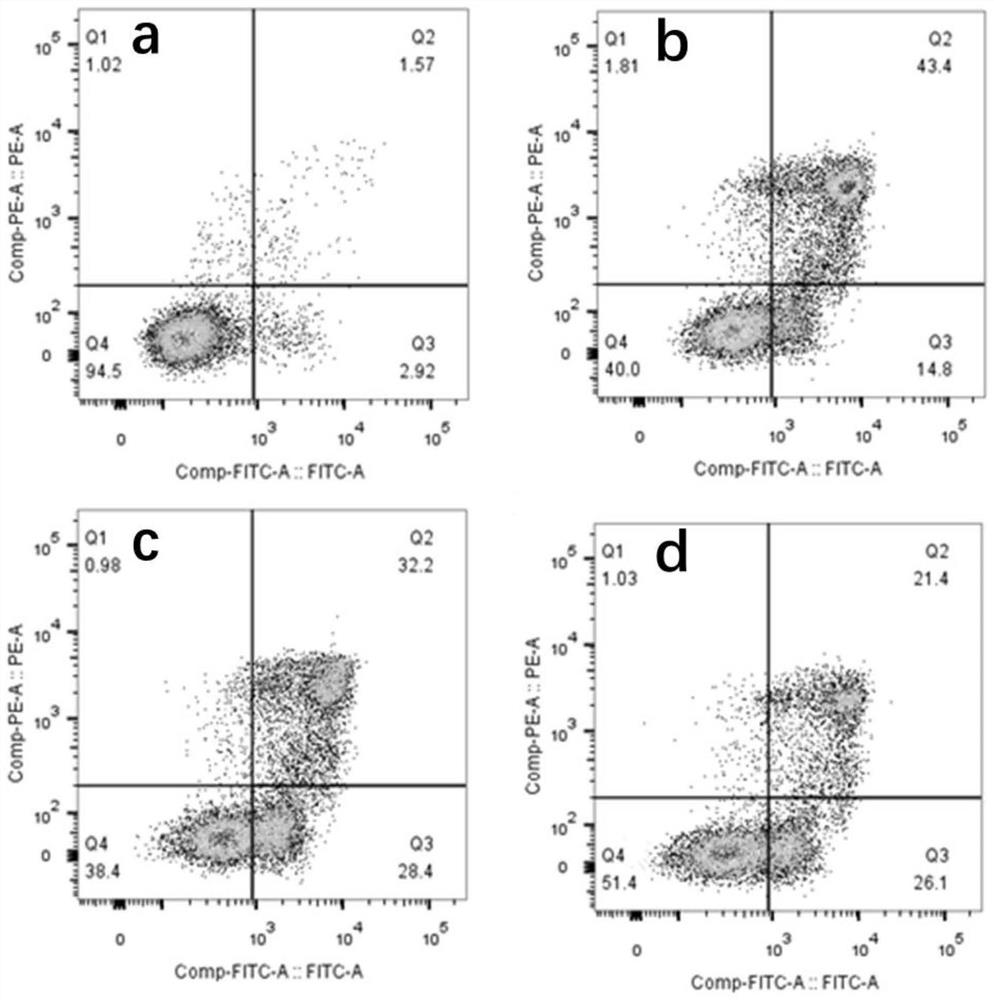
[0085] Effect of embodiment 1PP1 on NEC model intestinal epithelial cells
[0086] 1. Experimental materials
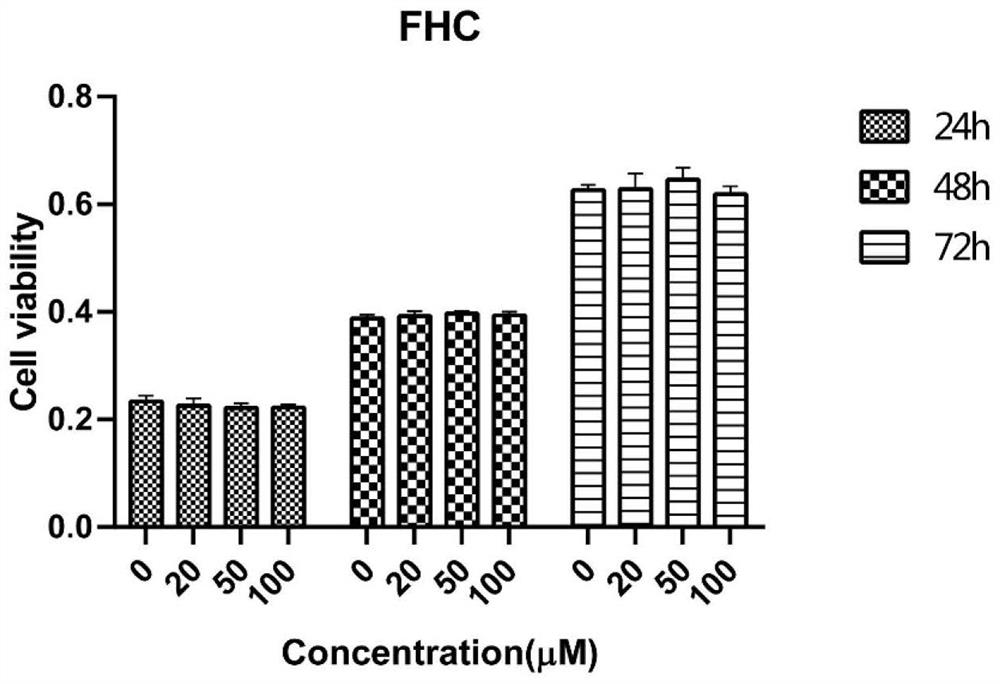
[0087] FHC cells: Fetal Human Colon Epithelial Cell, human intestinal epithelial cells, purchased from ATCC (CRL-1831).
[0088] 2. Experimental grouping
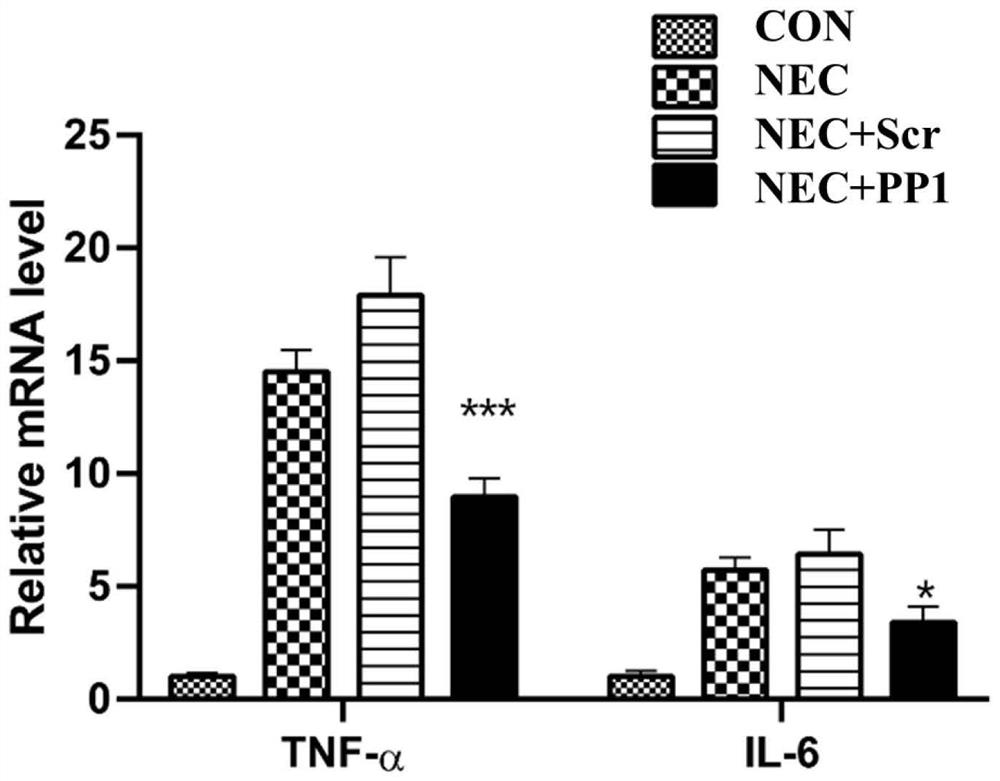
[0089] Set CON group, NEC group, NEC+Scr (scrambled polypeptide sequence) group and NEC+PP1 group.
[0090] 3. Experimental method
[0091] 3.1 Cell Culture
[0092] FHC cells at 37°C, 5% CO 2 Cultivate under conditions until the growth density is 80%-90%, to obtain FHC cell culture fluid.
[0093] 3.2 Construction of NEC cell model
[0094] CON group: No lipopolysaccharide (LPS) induction treatment was applied to the FHC cell culture medium;
[0095] NEC group: adding LPS (100 μg / mL) to the FHC cell culture medium to induce for 3 hours, and constructing the NEC cell model;
[0096] NEC+Scr group: add control peptide Scr (100 μM) to FHC cell culture medium, add LPS (100 μg / mL) after 1 hour to induce 3 h; ...
Embodiment 2
[0138] The impact of embodiment 2PP1 on NEC animal model
[0139] 1. Experimental animals
[0140] SPF-grade newborn SD rats and their mothers were purchased from the Animal Experiment Center of Nanjing Medical University, and they were raised in the Experimental Animal Center of Nanjing Medical University under the conditions of alternating light and dark every 12 hours, room temperature 24°C, and humidity 50%.
[0141] 2. Experimental grouping
[0142] Set CON group, NEC group, NEC+Scr group and NEC+PP1 group.
[0143] 3. Experimental method
[0144] 3.1 Construction of NEC animal model
[0145] Newborn SD rats weighing 6-9g within 24 hours of birth were randomly divided into four groups:
[0146] CON group: newborn SD rats were caged with their mothers and breast-fed;
[0147] NEC group: Neonatal SD rats were asphyxiated once every 8 hours (conditions: 5% O 2 , 95%N 2 , 5min), within 2min after the end of each hypoxia, hyperosmotic milk (Wyeth 1 stage milk powder: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com