Fluorescent polymer, Fe 3+ detection test paper and preparation method and application thereof
A fluorescent polymer and detection test paper technology, which is applied in the field of fluorescence detection, can solve the problems of expensive instruments and cumbersome operation process, and achieve the effects of simple detection, simple detection operation, and convenient processing and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A kind of fluorescent polymer, its structural formula is as follows:
[0043] Among them, n=60-120.
[0044] The synthetic route of above-mentioned fluorescent polymer is as follows:
[0045]
[0046] The preparation method of above-mentioned fluorescent polymer is as follows:
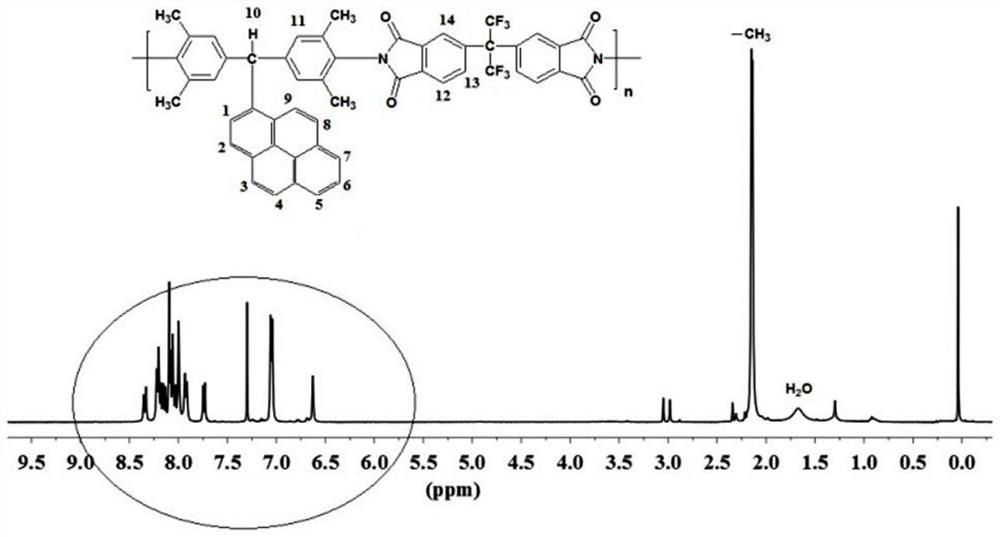
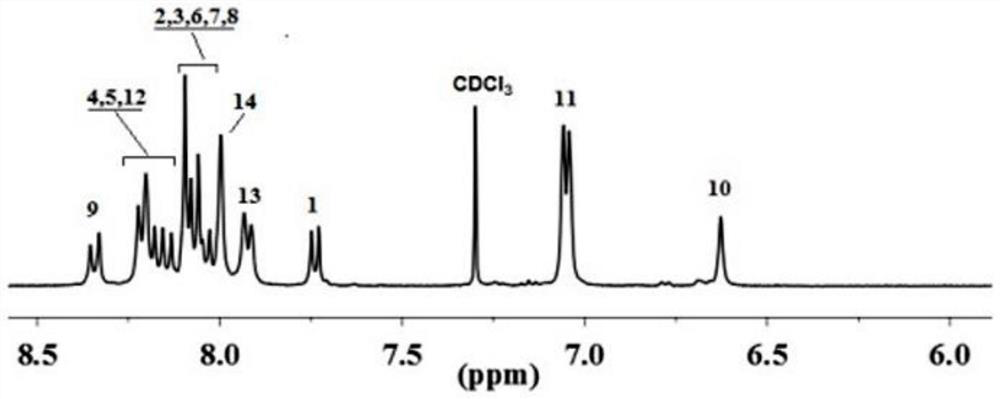
[0047] In a three-necked flask, add 5 mmol of diamine monomer and 5 mmol of hexafluorophthalic anhydride monomer, add 48 mL of cresol as solvent, 0.1503 g of isoquinoline as catalyst, stir magnetically at room temperature for 30 minutes, and then adjust the temperature of the reaction solution to 190 °C, the reaction was continued for 12 hours. After the reaction was completed, the reaction liquid was poured into 250 mL of absolute ethanol, and the reaction was completed by settling, further filtered and washed. Finally, the polymer was vacuum-dried at 120° C. for 10 hours to obtain the target polymer. figure 1 and figure 2 It is the NMR spectrum of the target polymer. It can be seen ...
Embodiment 2
[0049] a kind of Fe 3+ Detect the preparation of test paper, the preparation process is as follows:
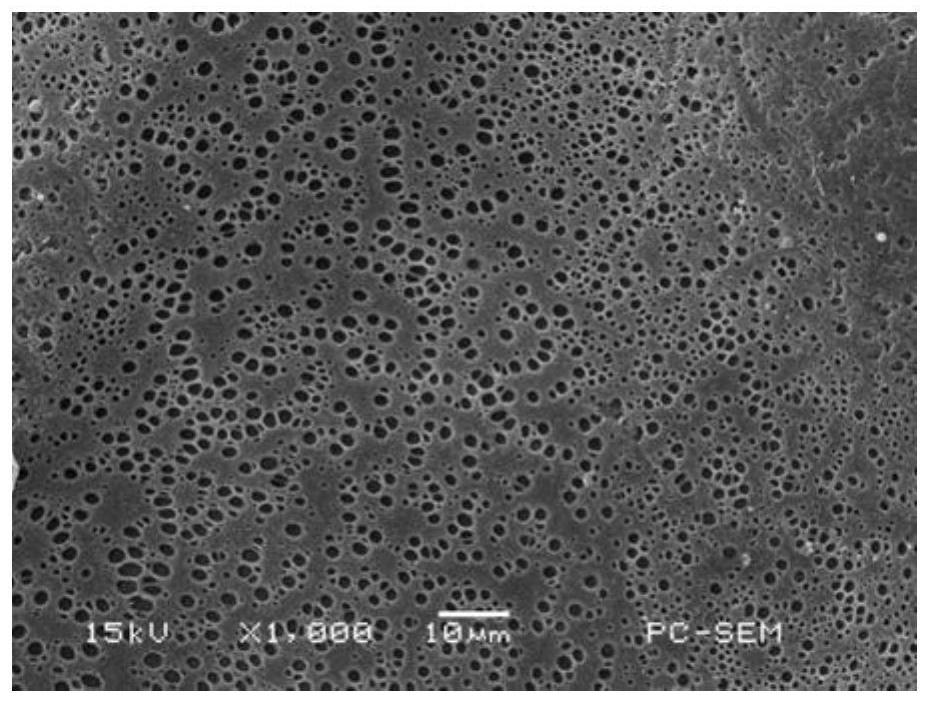
[0050] Weigh 1.041 g of the fluorescent polymer described in Example 1 and mix it with 10 mL of N,N-dimethylacetamide, shake until the polymer is fully dissolved, and prepare a 10 wt % fluorescent polymer solution. After immersing the filter paper in the polymer solution for 5 minutes, take it out and fix it on the support frame, and dry it at 80°C for 2 hours to obtain metal Fe 3+ Test strips.
[0051] Such as image 3 As shown, the prepared test paper has a smooth surface and a large number of pores, which are formed by the volatilization of the solvent during the drying process. These pores can allow the liquid to be tested to fully penetrate into the test paper, which is conducive to the adsorption of metal ions in the liquid.
Embodiment 3
[0053] Metal Fe 3+ The application of the detection test paper, the test process is as follows:
[0054] Prepare 5×10 -6 mol / L containing Fe 3+ Drop the solution on the detection test paper, wait for 60s, and compare it with the blank test paper under ultraviolet light irradiation.
[0055] Such as Figure 4 As shown, the detection test paper is adsorbing Fe 3+ The previous fluorescence emission spectrum shows the maximum fluorescence emission peak at 603nm, and the adsorption of Fe 3+ Afterwards, the maximum emission peak of the fluorescence red shifted to 630nm, and the test paper was absorbing Fe 3+ After the fluorescence intensity increased, compared with the ordinary filter paper under ultraviolet light and the test paper before and after adsorption, the detection test paper showed yellow fluorescence, and the adsorption of Fe 3+ After the test paper has obvious fluorescence enhancement phenomenon, it shows that the detection test paper prepared by the present inven...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com