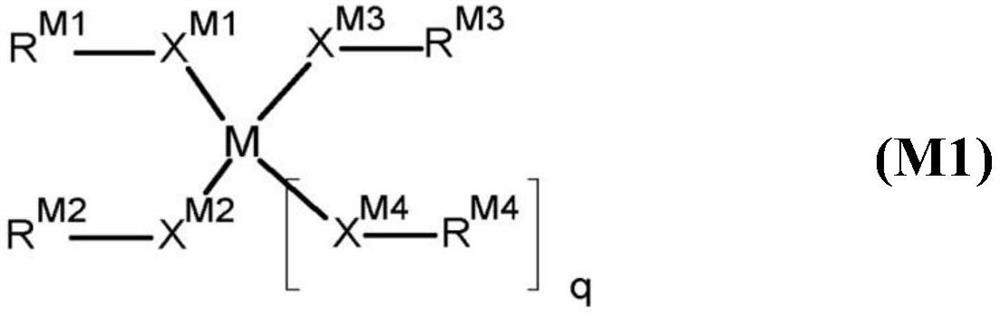
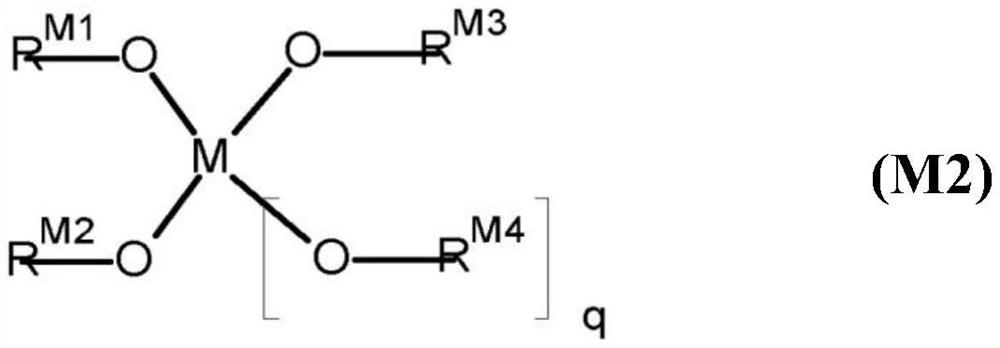
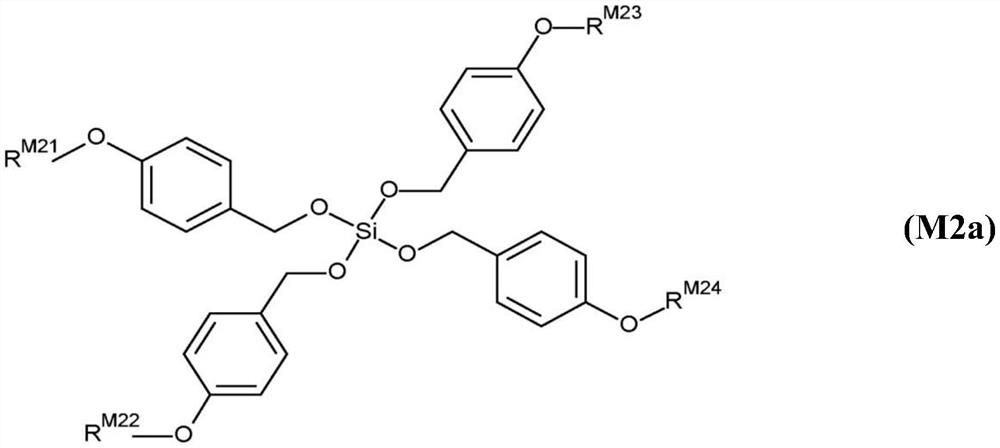
Twin-monomer composition and dielectric film thereof
A composition and monomer technology, applied in prepreg, dielectric film of Df and Dk, preparation of insulating film, application in multilayer printed circuit board and semiconductor device, dielectric film field, to achieve few layer defects , Uniform layer thickness, less layer inhomogeneity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0220] Measurement and evaluation of dielectric constant and loss tangent
[0221] The film thickness was measured with a micrometer (product of Mitutoyo, Japan, 0.001-5 mm). Dielectric measurements were done using a 10GHz Split Post Dielectric Resonator (SPDR) (product of QWED, Poland) and a vector network analyzer E5071C (product of keysight Technologies).
[0222] The SPDR was run in TE01δ mode, which confines the electric field component to the azimuthal direction of the membrane sample (F. Chen et al., Journal of Electromagnetic Analysis and Applications 4 (2012), 358–361). The resonance mode is insensitive to the air gap perpendicular to the membrane sample.
[0223] Dielectric constant D k Determined by the resonance frequency shift due to sample insertion. The typical uncertainty of the dielectric constant is better than ±1%, as the thickness measurement accuracy of the test sample is ±0.7% or better.
[0224] Loss tangent value D f The Q factors of the cavity and...
Embodiment A1
[0225] Example A1: Synthesis of tetra-(4-methoxybenzyloxy)silane
[0226] Under nitrogen, 108 g of 4-methoxybenzyl alcohol were dissolved in 500 ml of toluene in a 1 liter three-necked flask equipped with a mechanical stirrer. 82.1 g of 1-methylimidazole were added and 42.5 g of silicon tetrachloride were added slowly over 1 hour. The exothermic reaction was kept below 50 °C. Then, the mixture was heated at 100° C. for 5 hours with continuous stirring.
[0227] Stirring was stopped and the mixture was cooled to room temperature. The imidazolium salt formed was filtered and the solution was concentrated at 100° C. and 5 mbar. 112 g of product are obtained. 1 H-NMR (CD 2 Cl 2 ): 3.76ppm (s, 12H), 4.70ppm (s, 8H), 6.83ppm (d, 8H), 7.20ppm (d, 8H).
Embodiment A2
[0228] Embodiment A2: the synthesis of two-(4-methoxybenzyloxy)dimethylsilane
[0229] Under nitrogen, 69.1 g of 4-methoxybenzyl alcohol were dissolved in 400 ml of toluene in a 1-liter three-necked flask equipped with a mechanical stirrer. 41 g of 1-methylimidazole were added and 32.3 g of dichlorodimethylsilane were added slowly within 1 hour at 40°C. During the addition, the temperature rose to 50°C. The mixture was then heated to 60°C with continuous stirring for 0.5 hours, then at 85°C for an additional 2 hours.
[0230] Stirring was stopped and the mixture was cooled to room temperature. The imidazolium salt formed was filtered and the solution was concentrated at 90° C. and 10 mbar. 78 g of product are obtained. 1 H-NMR (CD 2 Cl 2 ): 0.16ppm (s, 6H), 3.77ppm (s, 6H), 4.66ppm (s, 4H), 6.85ppm (d, 4H), 7.22ppm (d, 4H). Example B1: Polymerization and Membrane Formation Using Silica and Acid Evaporation
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com