Protein stabilizer and preparation method thereof
A stabilizer and protein technology, applied in the biological field, can solve problems such as limited applicability, insufficient ability of protein stability, conflicts, etc., to achieve the effects of extending shelf life, extending stability, and reducing background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
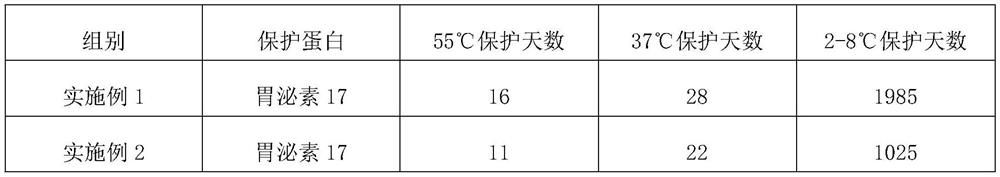
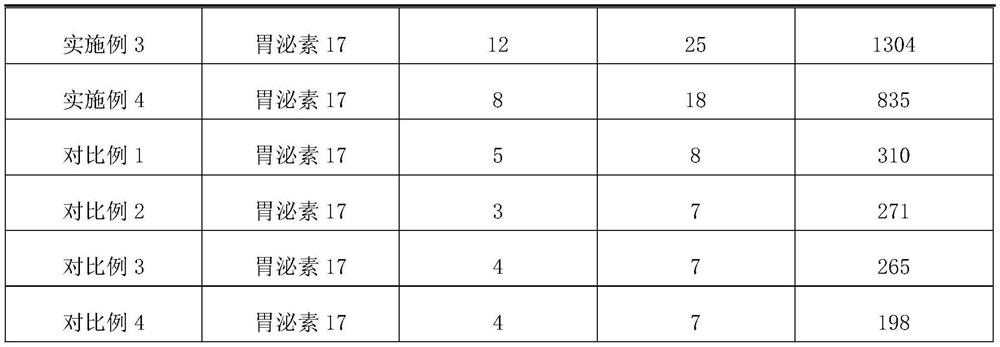
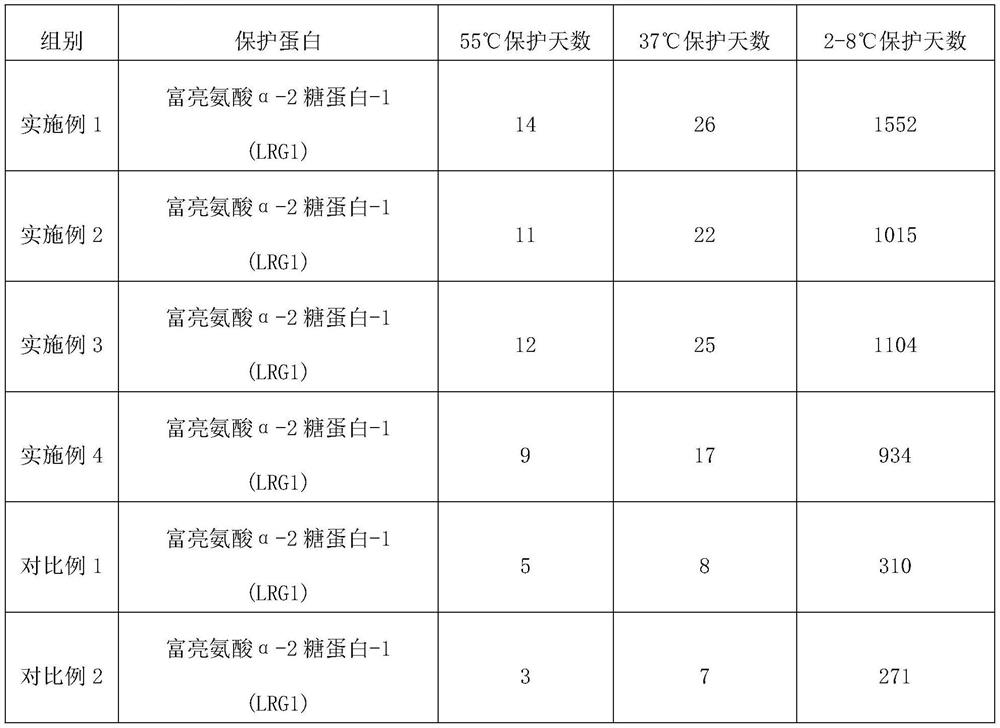
Embodiment 1
[0024] The protein stabilizer described in embodiment 1 comprises the raw materials of following parts by weight:
[0025] 0.5 part of high molecular weight hyaluronic acid, 0.1 part of medium molecular weight hyaluronic acid, 0.2 part of low molecular weight hyaluronic acid, 0.3 part of oligomeric hyaluronic acid, 0.05 part of acetylated hyaluronic acid, 0.3 part of sodium polyacrylate, ethylenediaminetetra 0.03 parts of disodium acetate dihydrate, 0.5 parts of phenylmethylsulfonyl fluoride, 0.5 parts of magnesium chloride, 0.1 parts of polyethylene glycol methyl ether, 0.09 parts of potassium chloride, 0.05 parts of ProClin950, 0.06 parts of ProClin 300, bovine serum albumin 0.2 parts, 6 parts of collagen, 0.3 parts of dipropylene glycol, phosphate buffer solution.
[0026] The preparation method of protein stabilizer described in embodiment 1, comprises the following steps:
[0027] (1) Add the above-mentioned components into the phosphate buffer solution, and mix and stir...
Embodiment 2
[0031] The protein stabilizer described in Example 2 includes the following raw materials in parts by weight: 0.5 part of high molecular weight hyaluronic acid, 0.1 part of medium molecular weight hyaluronic acid, 0.2 part of low molecular weight hyaluronic acid, 0.3 part of oligomeric hyaluronic acid, acetyl 0.05 part of hyaluronic acid, 0.3 part of sodium polyacrylate, 0.03 part of disodium edetate dihydrate, 0.5 part of phenylmethylsulfonyl fluoride, 0.5 part of magnesium chloride, 0.1 part of polyethylene glycol methyl ether, chlorinated Potassium 0.09, ProClin 950 0.05, ProClin 300 0.06, bovine serum albumin 0.2, collagen 6, glycerol 0.3, phosphate buffered saline.
[0032] The preparation method of protein stabilizer described in embodiment 2 may further comprise the steps:
[0033](1) Add the above-mentioned components into the phosphate buffer solution, and mix and stir until completely dissolved at a stirring speed of 1000 rpm;
[0034] (2) adjust the pH value betwee...
Embodiment 3
[0037] The protein stabilizer described in Example 3 includes the following raw materials in parts by weight: 0.4 part of high molecular weight hyaluronic acid, 0.8 part of medium molecular weight hyaluronic acid, 0.3 part of low molecular weight hyaluronic acid, 0.1 part of oligomeric hyaluronic acid, acetyl 0.6 part of hyaluronic acid, 0.5 part of sodium polyacrylate, 0.5 part of disodium edetate dihydrate, 1 part of phenylmethylsulfonyl fluoride, 0.2 part of magnesium chloride, 0.9 part of polyethylene glycol methyl ether, chlorinated Potassium 0.9 parts, ProClin 950 0.9 parts, ProClin 300 0.9 parts, bovine serum albumin 4 parts, collagen 7 parts, dipropylene glycol 1 part, Tris-HCl buffer solution.
[0038] The preparation method of protein stabilizer described in embodiment 3, comprises the following steps:
[0039] (1) Add the above-mentioned components into the Tris-HCl buffer solution, and mix and stir until completely dissolved at a stirring speed of 1000 rpm;
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com