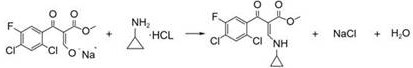
Preparation method of ciprofloxacin key intermediate
A technology for ciprofloxacin and intermediates, which is applied in the field of preparation of key intermediates of ciprofloxacin, can solve the problems of high cost, environmental protection pressure, high toxicity, low price, etc., and achieves avoiding three waste treatment costs and low production costs. , the effect of reducing the three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
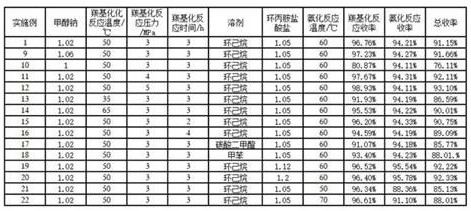
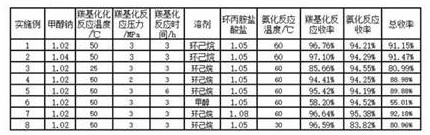
[0022] (1) Carbonylation reaction: Take a 250ml autoclave, add 20g of methyl 2,4-dichloro-5-fluorobenzoylacetate, 4.16g of solid sodium methoxide (2,4-dichloro-5-fluorobenzoyl 1.02 equivalents of methyl acetoacetate), 100 g of cyclohexane (the amount of solvent used is 5 times that of methyl 2,4-dichloro-5-fluorobenzoylacetate), and the kettle was closed. Use nitrogen 0.5MPa to replace the air in the kettle three times, use carbon monoxide to replace the nitrogen in the kettle three times, and fill the carbon monoxide pressure to 3.0MPa, raise the temperature in the water bath to the temperature in the kettle to 50°C, always pay attention to the pressure in the kettle and keep the pressure at 3.0MPa for 3 hours. . After the reaction was completed, cool to room temperature to carefully discharge carbon monoxide, cool to 10°C, filter with suction, rinse with 20g of methanol, and dry at 40°C to obtain 23.01g of (1Z)-3-(2,4-dichloro-5-fluorophenyl )-2-(methoxycarbonyl)-3-oxoprop-...
Embodiment 2
[0026] (1) Carbonylation reaction: take a 250ml autoclave, add 20g of methyl 2,4-dichloro-5-fluorobenzoylacetate, 4.24g of sodium methoxide solid (2,4-dichloro-5-fluorobenzoyl 1.04 equivalents of methyl acetoacetate), 100 g of cyclohexane (the amount of solvent used is 5 times that of methyl 2,4-dichloro-5-fluorobenzoylacetate), and the kettle was closed. Use nitrogen 0.5MPa to replace the air in the kettle three times, use carbon monoxide to replace the nitrogen in the kettle three times, and fill the carbon monoxide pressure to 3.0MPa, raise the temperature in the water bath to the temperature in the kettle to 50°C, always pay attention to the pressure in the kettle and keep the pressure at 3.0MPa for 3 hours. . After the reaction was completed, cool to room temperature to carefully discharge carbon monoxide, cool to 10°C, filter with suction, rinse with 20g of methanol, and dry at 40°C to obtain 23.09g of (1Z)-3-(2,4-dichloro-5-fluorophenyl )-2-(methoxycarbonyl)-3-oxoprop-...
Embodiment 3
[0030] (1) Carbonylation reaction: Take a 250ml autoclave, add 20g of methyl 2,4-dichloro-5-fluorobenzoylacetate, 4.16g of solid sodium methoxide (2,4-dichloro-5-fluorobenzoyl 1.02 equivalents of methyl acetoacetate), 100 g of cyclohexane (the amount of solvent used is 5 times that of methyl 2,4-dichloro-5-fluorobenzoylacetate), and the kettle was closed. Use nitrogen 0.5MPa to replace the air in the kettle three times, use carbon monoxide to replace the nitrogen in the kettle three times, and fill the carbon monoxide pressure to 3.0MPa, raise the temperature in the water bath to the temperature in the kettle to 25°C, always pay attention to the pressure in the kettle and keep the pressure at 3.0MPa for the reaction 3h. After the reaction was completed, cool to room temperature to carefully discharge carbon monoxide, cool to 10°C, filter with suction, rinse with 20g of methanol, and dry at 40°C to obtain 20.37g of (1Z)-3-(2,4-dichloro-5-fluorophenyl )-2-(methoxycarbonyl)-3-ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com