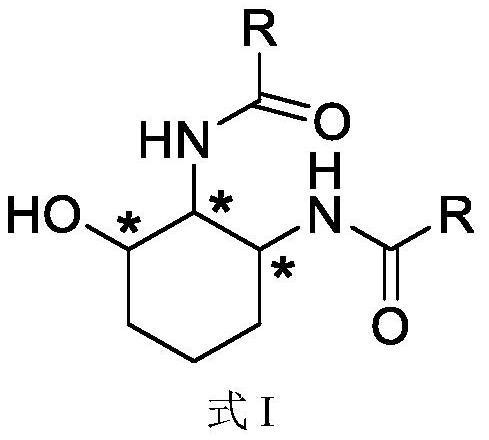
Hydroxyl cycloadipamide compound as well as preparation method and application thereof
A technology of hydroxycycloadipamide and compound, which is applied in the fields of antiviral infection drugs and chemical drugs, can solve problems such as instability, unstable structure, and easy hydrolysis, and achieve high stability, high anti-oxidation stability, and cell The effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
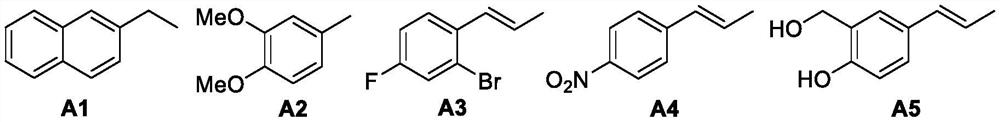
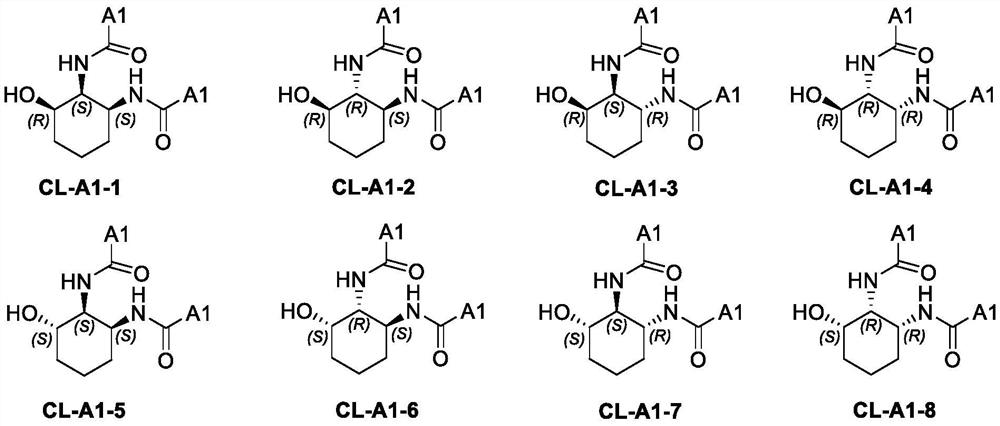
[0112] Preparation, separation and purification of 3-hydroxycyclohexyl-1,2-di(β-naphthyl)acetamide (CL-A1-1~CL-A1-8)
[0113] (1) Preparation of β-naphthylacetic acid: purchased through conventional market channels.
[0114] (2) Preparation of 2,3-diaminocyclohexyl-1-benzyl ether
[0115] 2.1. Preparation of 2-cyclohexen-1-ol
[0116] Measure 2-cyclohexen-1-one (0.49mL, 5mmol) in a dry 50mL round bottom flask, dissolve it with an appropriate amount of dry methanol, place the flask in a low-temperature constant temperature stirring reaction bath, and then weigh cerium trichloride (1.60g, 6.5mmol) was added to the flask, kept at -15°C and stirred for 0.5h, then slowly added sodium borohydride (245.92mg, 6.5mmol) in 3 portions within 15 minutes, and kept at -15°C for 3h. After the reaction is complete, slowly add 3mL of saturated ammonium chloride solution dropwise at -10°C to quench the reaction, then transfer the reaction solution to a separatory funnel, extract with DCM (3×5...
Embodiment 2
[0142] Preparation and Purification of 3-Hydroxycyclohexyl-1,2-bis(3,4-dimethoxyphenyl)formamide (CL-A2-1~CL-A2-8)
[0143] (1) Preparation of 3,4-dimethoxybenzoic acid. Buy from commercial sources.
[0144] (2) Preparation of 2,3-diaminocyclohexyl-1-benzyl ether. Prepare according to step 2.1 to step 2.6 of Example 1.
[0145] (3) Preparation and separation and purification of 3-hydroxycyclohexyl-1,2-bis(3,4-dimethoxyphenyl)formamide (CL-A2-1~CL-A2-8).
[0146] 3.1. Carry out according to step 3.1 of Example 1, wherein the raw material β-naphthaleneacetic acid is replaced by 3,4-dimethoxybenzoic acid (364.4mg, 2.0mmol), the amount of 2,3-diaminocyclohexyl-1-benzyl ether As (180.7mg, 0.82mmol). TLC developer condition is V DCM :V CH3OH =25:1, separated and purified by silica gel column chromatography, collected R f =0.61 fraction, 242.9mg of white solid was obtained, the yield was 54%. 1 H NMR (400MHz, CDCl 3 )δ8.17(d, J=9.1Hz, 1H), 7.84(d, J=8.6Hz, 1H), 7.14(dd, J=26...
Embodiment 3
[0159] Preparation and Purification of 3-Hydroxycyclohexyl-1,2-bis((E)-3-(2-bromo-4-fluorophenyl))acrylamide (CL-A3-1~CL-A3-8)
[0160] (1) Preparation of (E)-3-(2-bromo-4-fluorophenyl)acrylic acid
[0161] 1.1. Dissolve 2-bromo-4-fluorobenzaldehyde (203.0mg, 1.0mmol) and maleic acid (260.2mg, 2.5mmol) in 30mL of pyridine, then add a catalytic amount of piperidine (12.8mg, 0.15mmol), and the mixture Slowly raise the temperature to 110° C., and keep stirring at this temperature for 15 hours, followed by TLC until the end of the reaction. The reaction mixture was cooled to room temperature, and 30mL of 1M NaOH solution was added until the solution was in a clear state, washed with ethyl acetate (20mL×2), and the aqueous layer was adjusted to pH 2.0 with 50% sulfuric acid solution, a precipitate was precipitated, cooled in an ice bath, and suction filtered The solid compound was collected, washed successively with cold water and cold diethyl ether, sucked dry, and dried in vacuo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com