Method for screening glutamine transporter inhibitor, inhibitor screened by adopting method and application of inhibitor
A technique for glutamine and transporters, which is applied in the field of screening inhibitors of glutamine transporters, and can solve problems such as high cost, low throughput, and poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The construction of embodiment 1 plasmid
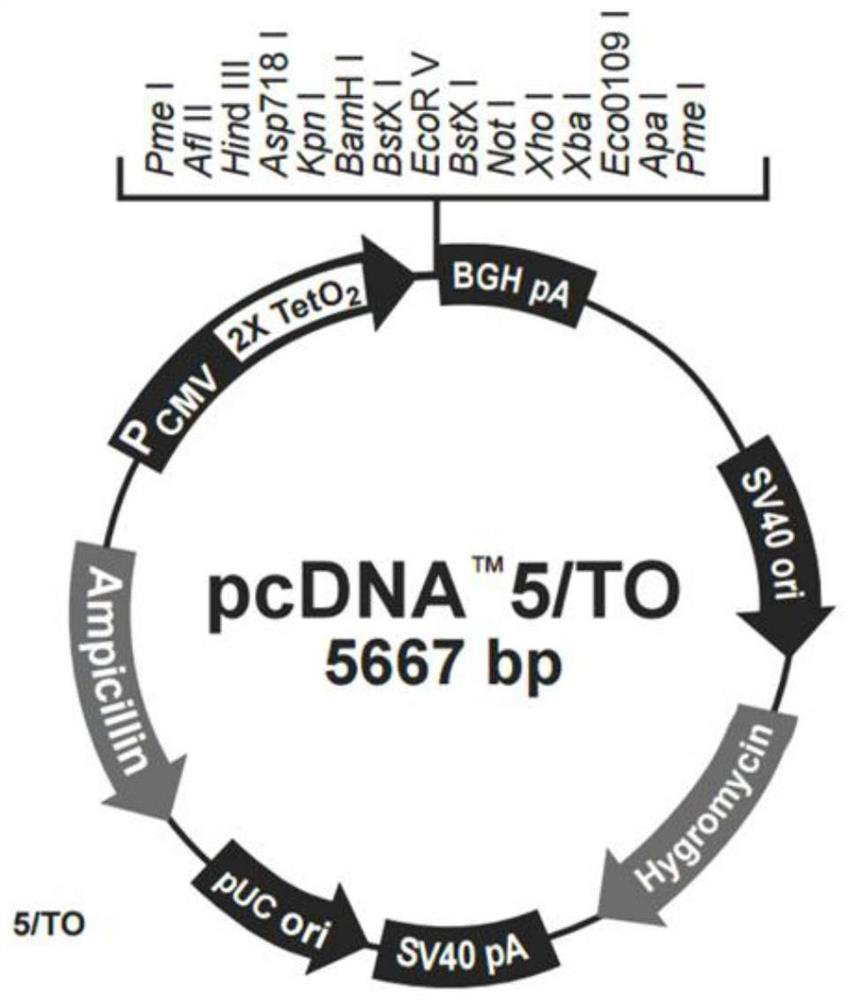
[0060] The hSLC1A5 gene sequence (NM_005628.3) was obtained from a gene bank, and the hSLC1A5 target gene was obtained by gene synthesis using synthetic biology methods, wherein the nucleotide sequence of the hSLC1A5 target gene was shown in SEQ ID No.1. The above-mentioned genes were cloned into the vector pcDNA5-TO (Hygromycin / Ampicillin) through 5'NotI and 3'XbaI (such as figure 1 shown), prepare the mini-scale recombinant plasmid DNA and the puncture bacteria containing the recombinant plasmid, and construct the pcDNA5-TO-hSLC1A5 plasmid for subsequent experiments.
Embodiment 2
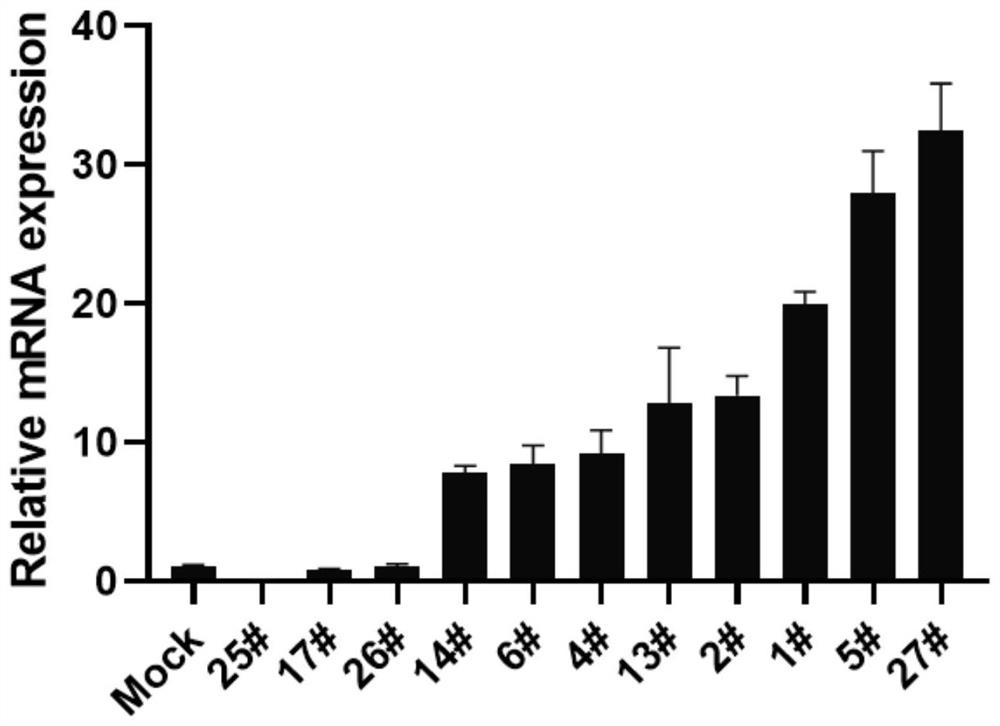
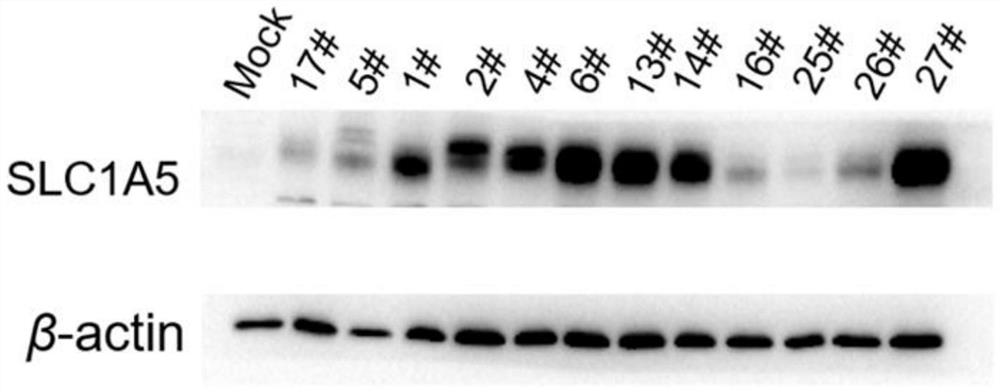
[0061] Example 2 Stable transfection cell line construction and verification
[0062] (1) Source and culture of cells:
[0063] The transfected cells were selected as human embryonic kidney cells (HEK293 cells), and the HEK293 cells were purchased from ATCC (American type culture collection). The cells were grown in a 100mm cell culture dish and passaged every three days at a ratio of 1:5. The medium was DMEM containing 10% FBS, and cultured in an incubator containing 5% carbon dioxide, 95% humidity, and 37°C.
[0064] (2) Antibiotic concentration screening:
[0065] HEK293 cells were seeded in 6-well plates, and 0, 4, 8, 16, and 32 μL of 50 mg / mL hygromycin B prepared in PBS were added to make the final concentrations 0, 100, 200, 400, 800, and 1000 μg / mL. For cell state, select the final screening concentration as 200 μg / mL and maintain the concentration as 100 μg / mL.
[0066] (3) Plasmid transfection:
[0067] HEK293 cells in the logarithmic growth phase were taken, spr...
Embodiment 3
[0084] Example 3 Condition Optimization of SLC1A5 Inhibitor Screening Experimental System
[0085] (1) LC-MS / MS method establishment for deuterated glutamine (L-glutamine-2,3,3,4,4-D5):
[0086] The transport function of HEK293-hSLC1A5-positive monoclonal cells was tested using the SLC1A5 transporter substrate deuterated glutamine (L-glutamine-2,3,3,4,4-D5) to evaluate the function of the monoclonal cell transporter. Firstly, a LC-MS / MS method capable of effectively determining the content of L-glutamine-2,3,3,4,4-D5 was developed, the chromatogram is as follows Figure 5 as shown, Figure 5 In , the standard concentration of deuterated glutamine is 10 μM. The conditions for LC-MS / MS analysis of the concentration of deuterated glutamine are as follows: the chromatographic column is VenusilHLP C185 μm (4×150mm); mobile phase A phase: water mixed with 10mM ammonium acetate containing 0.1% formic acid, B phase: acetonitrile, sample injection run time is 5min, elution mode is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com