Method for detecting ethyl p-toluenesulfonate and isopropyl p-toluenesulfonate in dabigatran etexilate bulk drug or preparation
A technology of isopropyl toluenesulfonate and ethyl toluenesulfonate, which is applied in the field of pharmaceutical analysis, can solve the problems of unreported content determination methods, and achieve the effects of high sensitivity, simple operation and accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] Preparation of blank excipient solution: Take the addition of accessories in the group in groups, hydroxypropylene, hydroxypropylcellulose, talc, and tartaric acid, mixed, plus the amount ratio of the proportion of the plug-in. 50% aqueous aqueous aqueous solution dissolved, shaken, filtered through 0.22 μm filtration membrane, and filtered into a blank excipient solution.
[0069] For ethyl p-toluenesulfonate, the formation of isopropyl toluenesulfonic acid rats: precision weighing toluenesulfonate, is about 10 mg of isopropyl toluenesulfonate, 25 ml of 10 ml of acetonitrile In the volumetric flask, add acetonitrile to the scale, shake well; 1 ml of 1ml of 1ML is taken at 100 mL of volumetric flask, add 50% aqueous aqueous solution of acetonitrile to the scale, shake well; re-eliminate 25 μl of 25mL volumetric flask, plus 50% The aqueous aqueous acetonitrile solution was diluted to the scale, shake, and obtained a solution of ethyl toluenesulfonate, the isopropyl ester con...
Embodiment 1
[0073] Example 1: Systematic Applicability
[0074] (1) Test method:
[0075] Toluene sulfonate was taken, the toluene sulfonate was used continuously injected 6-pin, recording ethyl toluenesulfonate, and isopropyl toluenesulfonic acid.
[0076] (2) Test results:
[0077] As shown in Table 1. Ethyl toluenesulfonate, the solution of toluenesulfonate is propyride, and the 6-pin RSD is 7.4%, 4.0% (standard regulations: 6-pin peak area RSD ≤ 10.0%), respectively. It indicates that the native spectrum system is good in sampling, and the systematic applicability meets the requirements.
[0078] Table 1: Example 1 System Applicability Test Results
[0079]
[0080]
Embodiment 2
[0081] Example 2: Exclusive
[0082] (1) Test method:
[0083] Samples of blank solution (50% acetonitrile aqueous solution), (Dabikitrile capsule) blank excipient solution, toluenesulfonate, isopropyl toluene sulfonic acid, Damika sulfonate .
[0084] Among them, the detection method of Dabiki group ester control solution is high performance liquid chromatography, column, flow rate, column temperature, injection volume, mobile phase A, mobile phase B, and gradient elution method as before; detection wavelength 226 nm.
[0085] (2) Test results:
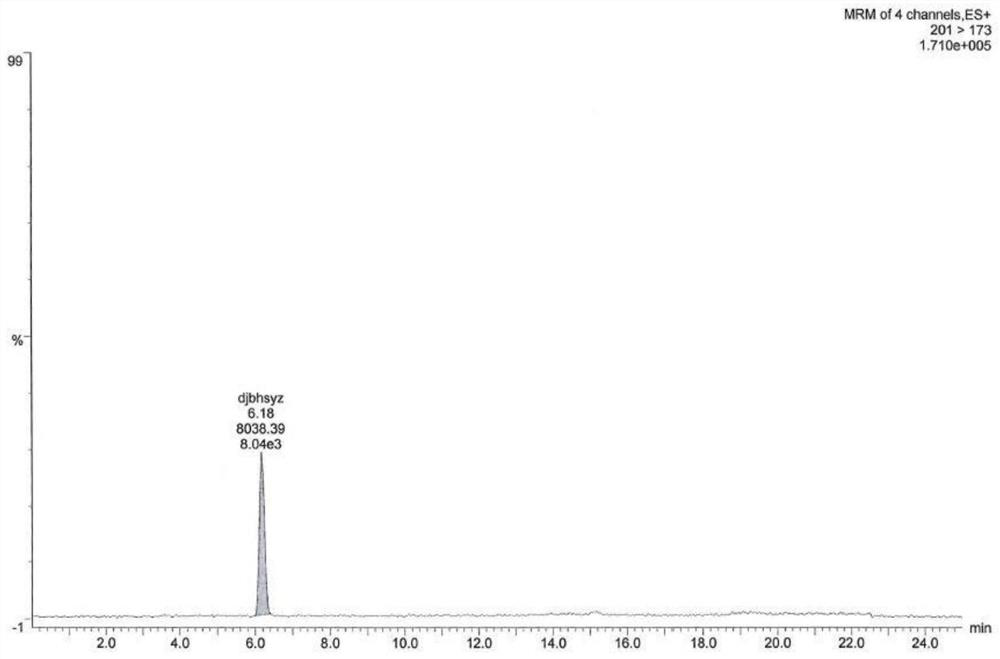
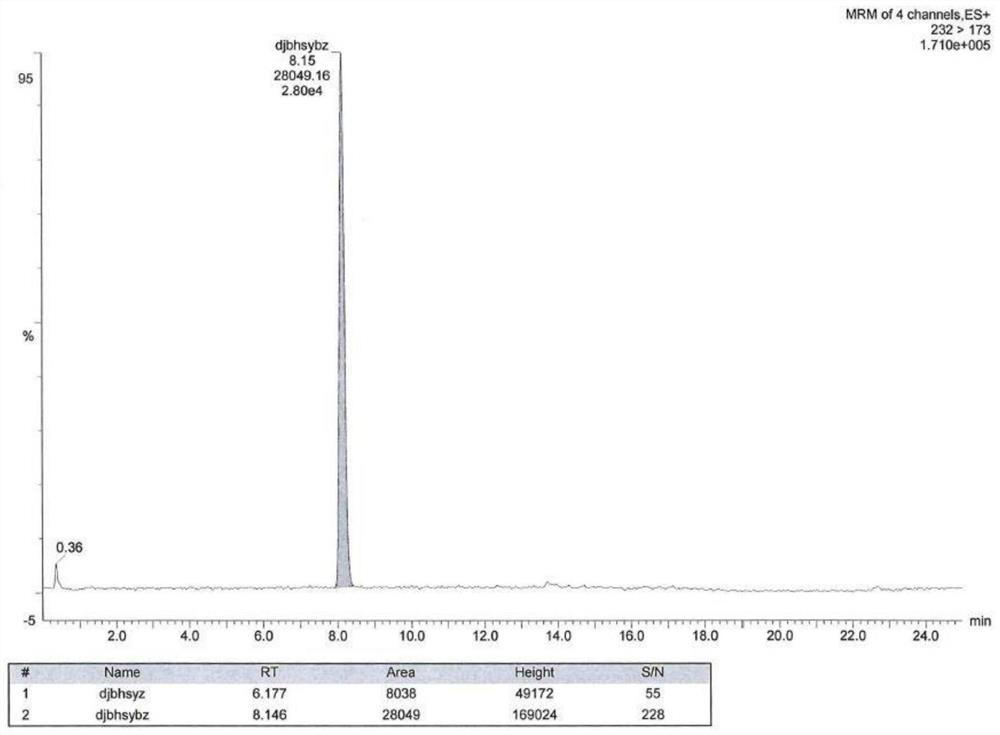
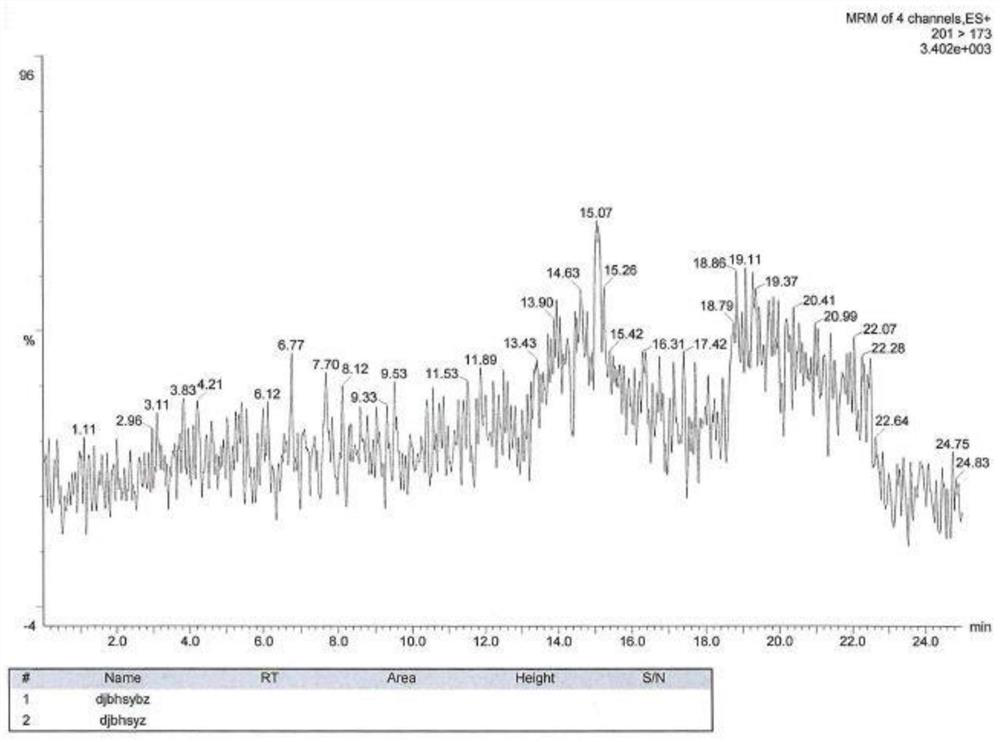
[0086] As shown in Table 2, a blank solution, a blank excipient solution, a ratio of ester pair of toluenesulfonate, and there is no interference with isopropyl toluenesulfonic acid. See Figure 1-7 .
[0087] Table 2: Example 2 Experimental test results
[0088]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com