Lipid nanoparticles containing pharmaceutical and/or nutraceutical agents and methods thereof
A nanoparticle, unsaturated fatty acid technology, applied in the field of nanoparticles, can solve problems such as active compound delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0174] Example 1. Solid lipid nanoparticle formulation of 4-(N)-docosahexaenoyl 2',2'-difluorodeoxycytidine with potent broad-spectrum antitumor activity.
[0175] The present example discloses a solid lipid nanoparticle (SLN) formulation comprising DHA-dFdC with improved apparent water solubility and chemical stability. SLN further comprised a lecithin / glyceryl monostearate emulsion in water emulsified with D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) and Tween 20. The resulting DHA-dFdC-SLN has a diameter of 102.2 ± 7.3 nm and increases the solubility of DHA-dFdC in water to at least 5.2 mg / mL, more than 200 times higher than its intrinsic water solubility. In comparison, the waxy solid of DHA-dFdC was unstable when stored at room temperature even in the presence of vitamin E as an antioxidant. However, DHA-dFdC in the lyophilized DHA-dFdC-SLN powder was not significantly degraded after one month of storage under the same conditions. DHA-dFdC-SLN also exhib...
example 2
[0213] Example 2. Oral SLN formulations with improved DHA-dFdC bioavailability.
[0214] Certain nanocarriers (e.g., SLNs, liposomes, nanoemulsions, micelles, and polymeric nanoparticles) improve drug resistance by increasing apparent drug solubility, reducing drug degradation in the gastrointestinal tract, and / or improving drug absorption. Oral delivery of cancer drugs, thus gaining some attention (Date, et al., J. Controlled Release, 2016, 240, 504-526; Thanki, et al., J. Controlled Release 2013, 170, (1), 15-40; Lin , et al., J. Food Drug Analysis, 2017, 25, (2), 219-234). This example discloses that DHA-dFdC-SLN surprisingly enables oral administration of a highly lipophilic compound, DHA-dFdC.
[0215] Materials and methods
[0216] Materials and cell lines. Mannitol, Tween 20, GMS, TPGS, sodium chloride (NaCl), hydrochloric acid (HCl, 37%), potassium dihydrogen phosphate (KH 2 PO 4 ), sodium hydroxide (NaOH), and Tween 80 were from Sigma-Aldrich (St. Louis, MO). Ge...
example 3
[0244] Example 3. Additional SLN formulations.
[0245] DHA-dFdC with different concentrations of TPGS
[0246] DHA-dFdC (5 mg), 3.5 mg soybean lecithin, 0.5 mg glyceryl monostearate and different amounts (0.4375 mg, 0.875 mg or 1.75 mg) of TPGS were mixed and dispersed in 800 μl deionized and filtered (0.22 μm) in hot water (80°C). The mixture was vortexed, sonicated for 10 min, and then kept on a hot plate at 80 °C while stirring at 800 rpm for 5 min. Separately, 55 mg of Tween 20 was dissolved in 1 ml of hot water, and 200 ml of this solution was added dropwise to the mixture until the final concentration was 1% (v / v) Tween 20. The emulsion was cooled to room temperature while stirring to form nanoparticles.
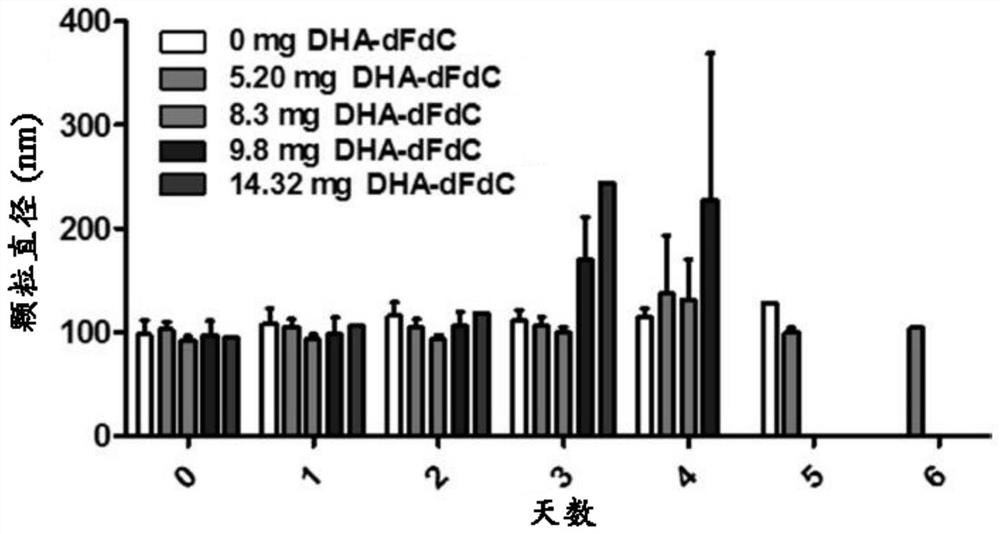
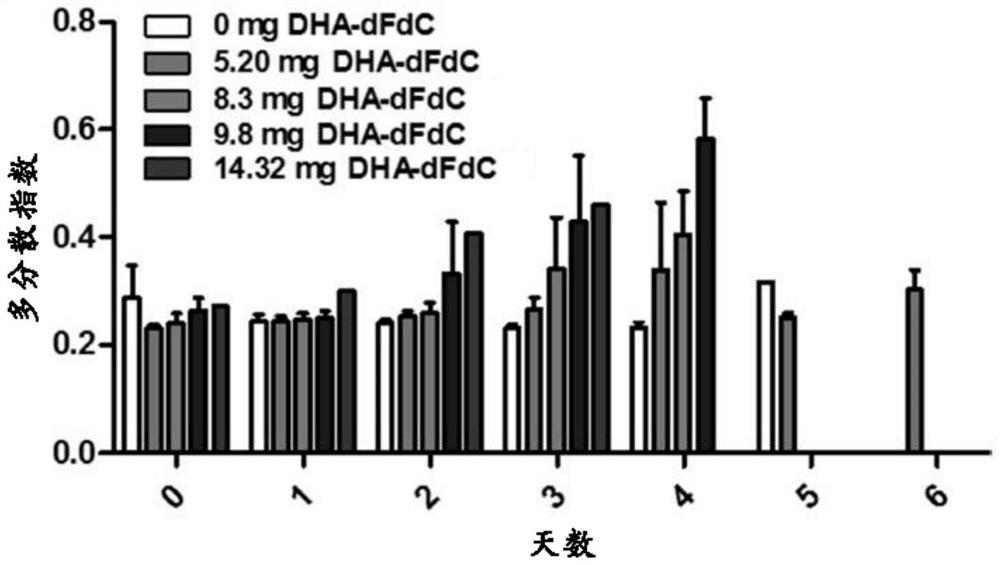
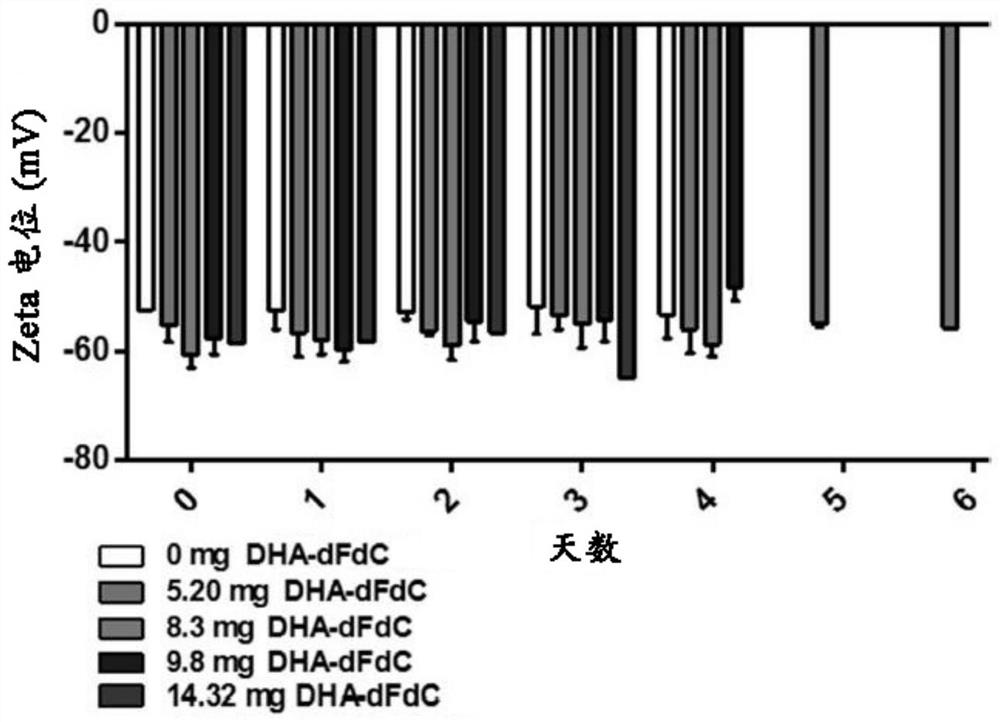
[0247] The particle diameter, polydispersity index (PDI) and zeta potential of the nanoparticles were determined using a Malvern Zeta Sizer Nano ZS (Westborough, MA). The results are summarized in Figures 12A-12C and Table 5. Nanoparticles prepared with 0.4375m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com