Silicate-carbonate composite mineral material, preparation method thereof and application of silicate-carbonate composite mineral material in precipitation of heavy metal ions
A technology of heavy metal ions and carbonate minerals, applied in chemical instruments and methods, flocculation/sedimentation water/sewage treatment, water pollutants, etc., can solve problems such as long settling time, failure to achieve effective fixation, and increased process costs , to achieve the effects of easy industrial production, remarkable precipitation effect, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of silicate-carbonate composite mineral material, its preparation method is as follows:
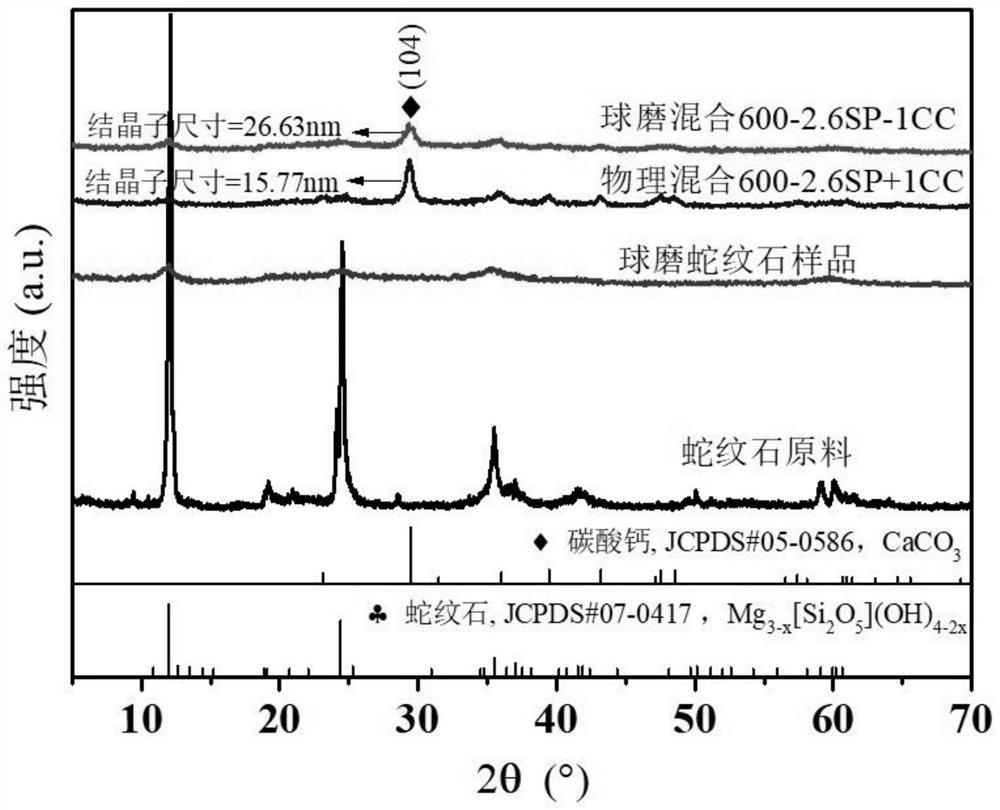
[0035] Weigh four groups of serpentine powder and calcium carbonate powder (CaCO 3 , abbreviated as CC, analytically pure): 1.6933+0.3067g (mass ratio SP: CC=5.2:1), 1.4681+0.5319g (mass ratio SP: CC=2.6:1), 1.2958+0.7042g (mass ratio SP: CC=1.7:1), 1.1597+0.8403g (mass ratio SP:CC=1.3:1), put them into 45mL zirconia grinding pots respectively, add 70g of zirconia balls with a diameter of 15mm in each group, the ball-to-material ratio 35:1, the mill speed was set to 600rpm, and each group of powder samples were collected after grinding for 60 minutes to obtain 4 kinds of serpentine-calcium carbonate composite material fixatives, which were marked as 600-5.2SP-1CC, 600-2.6SP -1CC, 600-1.7SP-1CC, 600-1.3SP-1CC.
[0036] Control group: take 2g each of serpentine and calcium carbonate, put them into a 45mL zirconia grinding jar, add 70g of zirconia balls with a diameter of 15m...
Embodiment 2
[0046] A silicate-carbonate composite mineral material, the preparation method of which is as follows:
[0047] Weigh 1.5g of olivine powder and 0.5g of calcium carbonate powder (mass ratio MSi:CC=2.6:1), put them into a 45mL zirconia grinding tank and add 70g of zirconia balls with a diameter of 15mm, the ball-to-material ratio is 35 : 1, the mill speed was set to 600rpm, and the powder samples were collected after grinding for 60min to obtain the olivine-calcium carbonate composite material fixative, marked as 600-2.6MSi-1CC.
[0048] Comparative sample: Weigh 2g each of olivine and calcium carbonate, put them into a 45mL zirconia grinding tank for ball milling, add 70g of zirconia balls with a diameter of 15mm each during the ball milling process, the ball-to-material ratio is 35:1, and grind The rotating speed of the machine is set to 600rpm, and the powder samples are collected after grinding for 60min to obtain ground and activated olivine and activated calcium carbonate...
Embodiment 3
[0055] A silicate-carbonate composite mineral material, the preparation method of which is as follows:
[0056] Weigh 1.5g of serpentine powder and 0.5g of dolomite powder (mass ratio SP:MCC=3:1), put them into a 45mL zirconia grinding tank, and add 70g of zirconia balls with a diameter of 15mm at the same time. The ratio was 35:1, the mill speed was set at 600rpm, and the powder sample was collected after grinding for 60min to obtain a serpentine-dolomite composite fixative, marked as 600-3SP-1MCC.
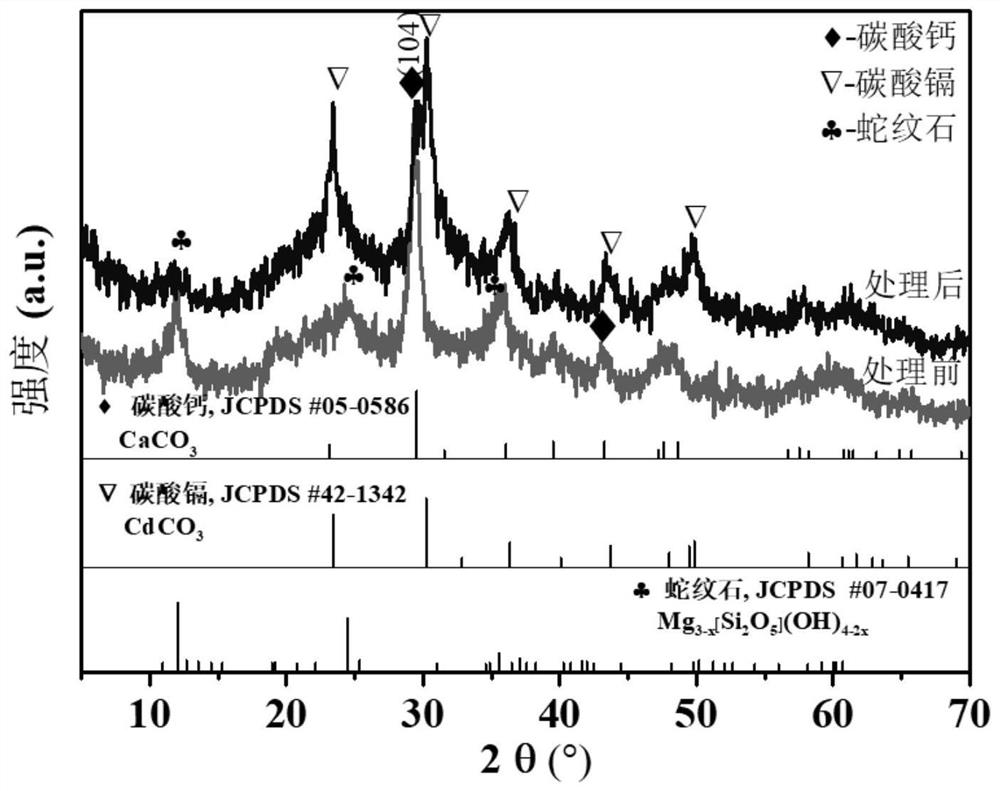
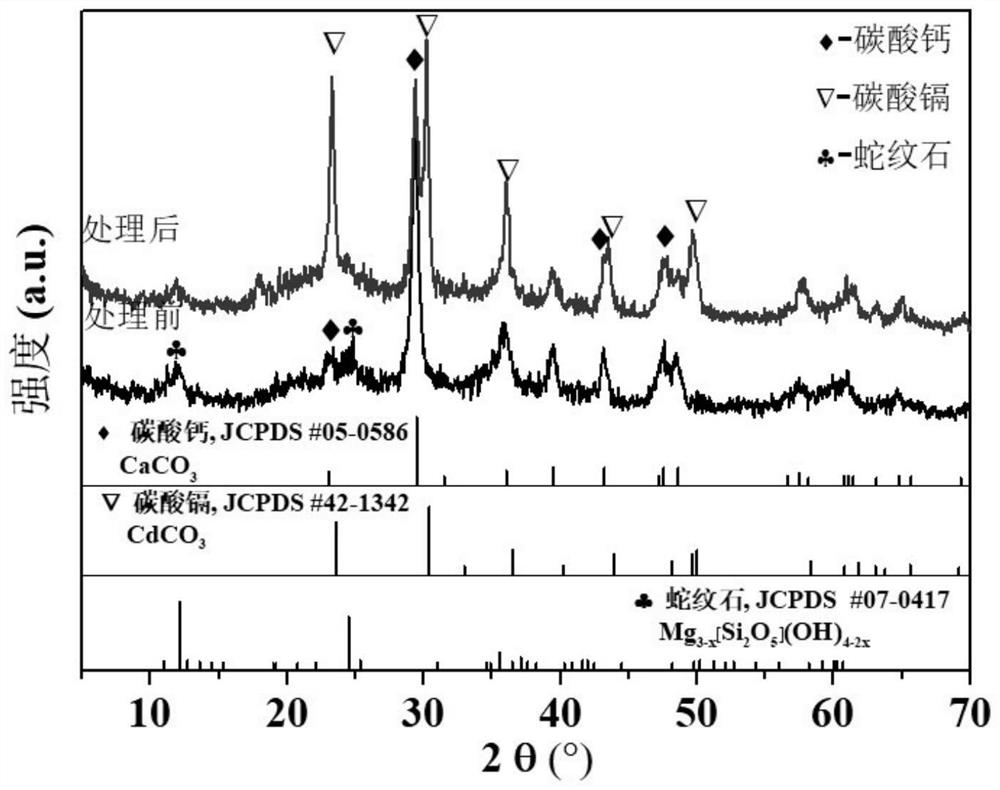
[0057] Using cadmium sulfate (CdSO 4 8 / 3H 2 O) The agent is formulated to contain Cd 2+ For a solution with a concentration of 100mg / L, take 100mL and place it in a beaker, press CO 3 2- with Cd 2+ The molar ratio is 1:1 as the standard, add 0.0346g of the fixative obtained in this example to the cadmium-containing solution and carry out the shaking and stirring reaction. The Cd element concentration and pH value in the medium, the experimental results are shown in Table 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com