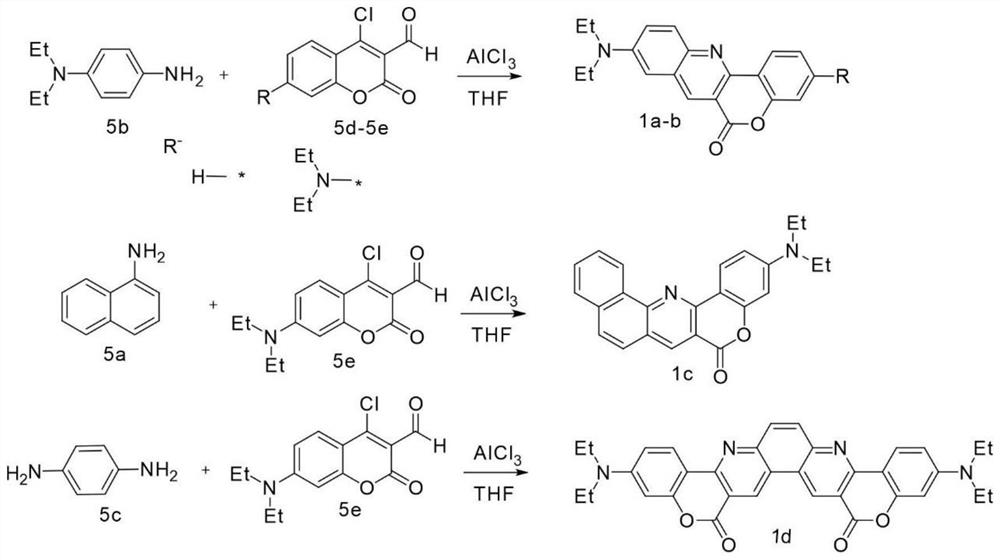
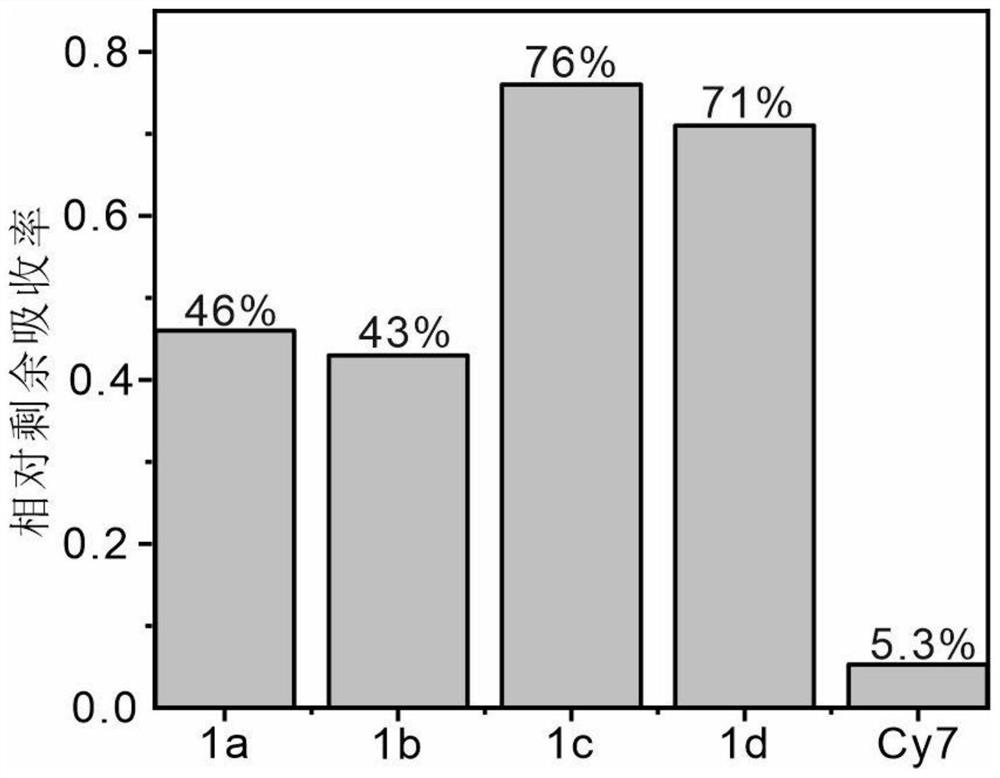
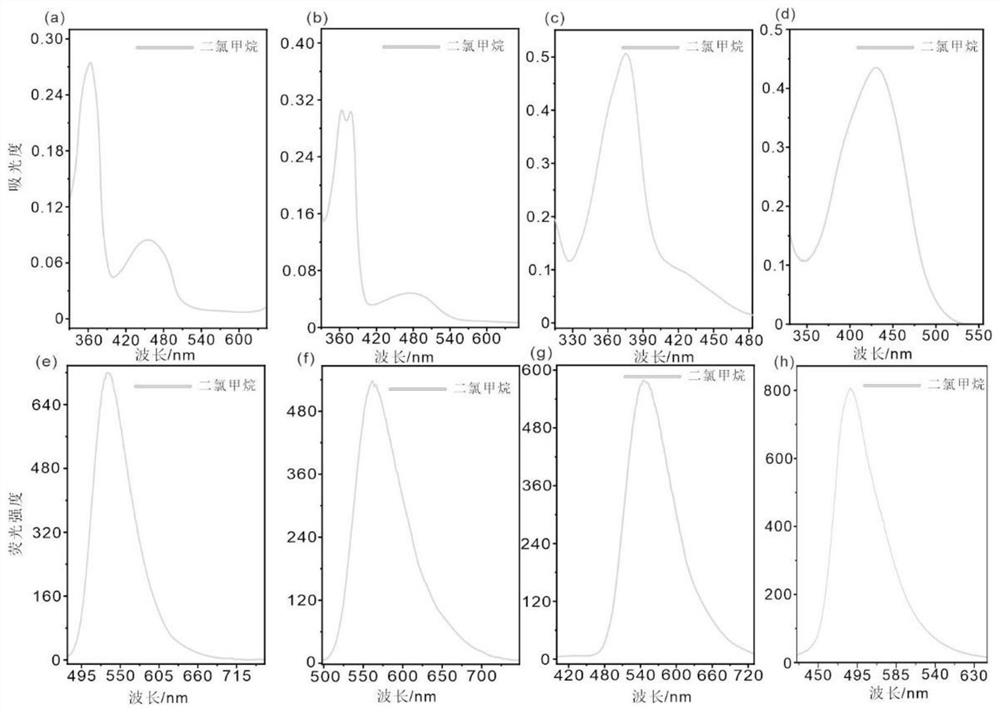
Amino-substituted chromene quinoline type fluorescent marker as well as preparation and application thereof
A fluorescent marker, quinoline-type technology, applied in the field of fluorescent labeling, can solve problems affecting the physiological activities of living organisms, lack of specificity of lipid droplets, changes in microenvironment, etc., and achieve great scientific significance and commercial value, Stokes Displacement background interference is small, easy to operate and effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The steps of dye 1a synthesis are as follows:
[0035] Take compound N,N-diethyl-p-phenylenediamine 5b (1.0 mmol, 393.0 mg), compound 5d (1.0 mmol, 500.0 mg) and aluminum chloride (1.0 mmol, 319.0 mg) dissolved in 20.0 ml In tetrahydrofuran, then refluxed for 3 to 4 hours, and TLC detected the reaction progress; after cooling down to room temperature, the solvent was removed directly by a rotary evaporator, and the pure dye 1a was obtained by column chromatography (eluent: pure dichloromethane). Orange solid, 175.3 mg, 44.5% yield. Characterized by: H NMR 1H NMR (300MHz, DMSO-d 6 )δ(ppm)8.97(s,1H,Ar-H),8.57(s,1H,Ar-H),8.58(d,J=5.7Hz,1H,Ar-H),8.02(d,J=9.6 Hz, Ar-H), 7.68(d, J=8.1Hz, 1H, Ar-H), 7.56(t, J=14.4Hz, 1H, Ar-H), 7.42(d, J=8.7Hz, 2H, Ar-H), δ7.20(s, 1H, Ar-H), 3.53(q, J=6.0Hz, 4H, 2×CH 2 ),2.50(s,6H,2×CH 3 ).Carbon NMR 13 C NMR (151MHz, CDCl 3 )δ (ppm) 161.9, 151.9, 144.9, 137.4, 130.8, 130.2, 123.1, 117.1, 115.8, 103.8, 44.7, 12.4. High resolution mass ...
Embodiment 2
[0037] The steps of dye 1b synthesis are as follows:
[0038] Take compound N,N-diethyl-p-phenylenediamine 5b (1.0 mmol, 176.16 mg), compound 5e (1.0 mmol, 300.0 mg) and aluminum chloride (1.0 mmol, 142.9 mg) dissolved in 20.0 ml In tetrahydrofuran, then refluxed for 3 to 4 hours, TLC detection reaction progress; after cooling down to room temperature, directly remove solvent by rotary evaporator, and obtain pure dye 1b through column chromatography (eluent: pure dichloromethane) separation, Orange-red solid, 66.8 mg, 23% yield. It is characterized by: NMR spectrum 1 HNMR (300MHz, DMSO-d 6 )δ (ppm) 8.87 (s, 1H, Ar-H), 8.29 (d, J = 6.6Hz, 1H, Ar-H), 7.91 (d, J = 7.2Hz, 1H, Ar-H), 7.60 ( d,J=6.6Hz,Ar-H),7.13(s,1H,Ar-H),6.77(t,J=6.6Hz,1H,Ar-H),6.56(s,1H,Ar-H), 3.46(q, J=18Hz, 8H, 4×CH 2 ), 1.17(q, J=6.0Hz, 12H, 4×CH 3 ).Carbon NMR 13 C NMR (151MHz, CDCl 3 )δ (ppm) 162.7, 153.9, 145.5, 137.9, 129.5, 128.3, 125.4, 123.0, 114.8, 108.9, 104.6, 98.1, 44.7, 12.6. High resolutio...
Embodiment 3
[0040] The steps of dye 1c synthesis are as follows:
[0041] Get compound 1-naphthylamine 5a (1.0 mmol, 153.6 mg), compound 5e (1.0 mmol, 300 mg) and aluminum chloride (1.0 mmol, 142.9 mg) are dissolved in 20.0 ml of THF, then reflux for 3 After 4 hours, TLC detected the reaction progress; after cooling down to room temperature, the solvent was removed directly by a rotary evaporator, and purified dye 1c was obtained by column chromatography (eluent: pure dichloromethane) separation, bright yellow solid, 123.7 mg, Yield 41.2%. It is characterized by: NMR spectrum 1 H NMR (300MHz, DMSO-d 6 )δ (ppm) 9.33 (d, J = 5.4Hz, 1H, Ar-H), 9.01 (s, 1H, Ar-H), 8.53 (d, J = 6.9Hz, 1H, Ar-H), 8.04 ( d,J=5.4Hz,Ar-H),7.98(d,J=6.6Hz,1H,Ar-H),7.84(q,J=9.0Hz,3H,Ar-H),6.83(d,J= 6.3Hz, 1H, Ar-H), 6.56(s, 1H, Ar-H), 3.46(q, J=5.1Hz, 4H, 2×CH 2 ), 1.16(q, J=9.9Hz, 6H, 2×CH 3 ).Carbon NMR 13 C NMR (151MHz, CDCl 3 )δ (ppm) 162.3, 154.7, 150.5, 149.8, 138.9, 135.1, 130.8, 129.7, 127.8, 127.0, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com