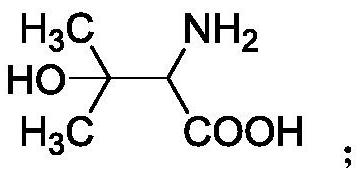
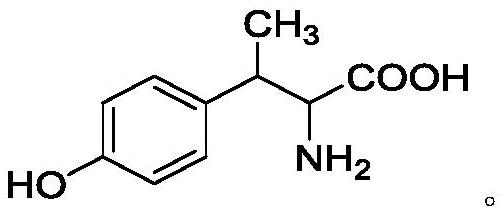
Application of 2-amino-3-hydroxy-3-methylbutyric acid and/or 2-amino-3-(4-hydroxyphenyl) butyric acid
A technology of methyl butyric acid and hydroxyphenyl, which is applied in the field of agricultural biopesticides to achieve the effect of simple structure and convenient biological extraction method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 (biological synthesis, extraction method and structural identification of the compound of the present invention)
[0047] (1) Cultivation of Alternaria
[0048] Glucose sodium nitrate medium: glucose, 40.0g; NaNO 3 , 1.0g; NH 4 Cl, 0.25g; KH 2 PO 4 , 1.0g; KCl, 0.25g; NaCl, 0.25g; MgSO 4 ·7H 2 O, 0.5g; FeSO 4 ·7H 2 O, 0.01g; ZnSO 4 ·7H 2 O, 0.01g; yeast extract, 1g, add water to 1L, adjust pH to 5.5.
[0049] The Alternaria culture method is as follows: PDA medium activates the preserved strains, and after 7 days, select colonies with consistent growth, take a 5mm diameter bacterial cake, and inoculate it into 500mL medium, and the inoculation amount is one bacterial cake per 100mL. Place the culture medium inoculated with the bacterial blocks into a constant temperature shaker, and the culture conditions are: 140 rpm, 25° C., and culture in the dark for 7 days.
[0050] (2) Extraction of compounds
[0051] Mycelia were isolated from the ferment...
Embodiment 2
[0063] The mass spectrum shows that the molecular ion peak of the compound is: 196.0967[M+H] + , determine its molecular formula as: C 10 h 13 NO 3 . Combined with the results of H NMR and C NMR, it was determined that the compound was 2-amino-3-(4-hydroxyphenyl)butyric acid. Example 2 (2-amino-3-hydroxyl-3-methylbutyric acid and 2-amino-3-(4-hydroxyphenyl) butyric acid induce tobacco resistance to tomato spotted wilt virus infection)
[0064] Tomato spotted wilt virus was obtained from Yunnan Province, China. The initial virus source was stored in a -80°C refrigerator. The virus was inoculated on the leaves of Nicotiana benthamiana by friction inoculation to activate the virus, and the virus plasmid was extracted using Escherichia coli competent cells. Transformation, smeared on resistant plates and cultured, picked a single colony for PCR screening, selected positive colonies for sequencing and subsequent plasmid extraction, added the plasmids with normal sequencing to A...
Embodiment 3
[0082] Example 3 (2-amino-3-hydroxy-3-methylbutyric acid and 2-amino-3-(4-hydroxyphenyl) butyric acid induce Arabidopsis resistance to Pseudomonas syringae infection)
[0083] Dissolve 2-amino-3-hydroxy-3-methylbutyric acid in sterile water, and then use sterile water to gradiently dilute it into 100nM, 1000nM and 10000nM solutions. A blank control is also set up, and 0.02% Tween 20 is added at the same time as Surfactant. Spread Pseudomonas syringae PstDC3000 on an LB plate and culture at 28°C for 48 hours; pick a single colony and inoculate it into a 50mL centrifuge tube containing 2mL of medium, culture it on a shaker at 28°C and 250rpm, monitor every 1-2h Bacteria solution OD 600 value change, at OD 600 Stop culturing bacteria before the value reaches 0.8; transfer 1mL of bacterial liquid to a sterile 1.5mL centrifuge tube, centrifuge at 8000rpm for 2min, and collect the precipitate; remove the supernatant, wash the precipitate with 10mM magnesium chloride for 3 times an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com