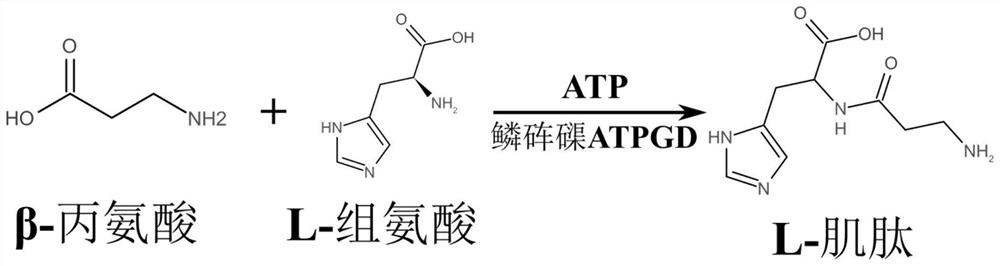
L-carnosine synthetase ATPGD derived from novel shellfish and application of L-carnosine synthetase ATPGD
A technology for synthesizing enzymes and carnosine, applied in the field of L-carnosine synthetase ATPGD, which can solve problems such as environmental pollution and complex routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0023] The acquisition of embodiment 1 ATPGD full-length gene
[0024] 1. RNA extraction
[0025] Total RNA was extracted using Trizol (Invitorgen), and the specific steps were as follows: (1) Put 50 mg of Tridacna gill tissue into a centrifuge tube with 1 ml of Trizol solution, grind thoroughly, and place at room temperature for 10 min; (2) Add 200 μL of chloroform, vigorously Shake for 40 seconds, and place at room temperature for 5 minutes; (3) Centrifuge at 12,000×g for 10 minutes at 4°C, and transfer the supernatant to a new tube; (4) Add 0.5ml of isopropanol, mix well, and let stand at room temperature for 10 minutes; (5) 4 Centrifuge at 12,000×g for 10 minutes at ℃, discard the supernatant; (6) wash the precipitate twice with 1ml 75% ethanol, and centrifuge at 12,000×g for 10 minutes at 4℃; RNA was stored at -80°C for later use. All containers and pipette tips in the above steps have been treated without RNAase.
[0026] 2. Reverse transcription and ATPGD full-length...
Embodiment 2
[0029] Prokaryotic expression of embodiment 2 recombinant protein
[0030] 1. Prokaryotic expression vector construction
[0031] (1) Design a pair of primers covering the ATPGD ORF of giant clam (F: 5'-ATGACGAGTTTCCGTGAAAGGTTCG-3'; R: 5'-CTAATCAGTCGTATGAGATGAAGAAGGCTC-3'); (2) Use this pair of primers for PCR amplification (reaction system : 0.5 μl of 10-fold diluted gill tissue cDNA, 1 μl of each primer (10 mM), 12.5 μl of Premix TaKaRaEx Taq (TaKaRa), 10 μl of ddH2O. Reaction program: 95°C for 3min; 95°C for 15s, 60°C for 15s, 72°C for 3min; ℃ 5min). After the PCR product was detected to meet the expected size by 1.5% agarose gel, it was recovered and purified using HiPure Gel Pure DNA Mini Kit (Magen), and the product was stored at -20°C after sequencing verification. (3) Using the PCR product as a template, use a primer pair (5'-CCGCGTGGATCCCCGGAATTCATGACGAGTTTCCGTGAAAGGT-3'; 5'-GTCACGATGCGGCCGCTCGAGCTAATCAGTCGTATGAGATGAAGAAGG-3') to amplify again and add a recombinatio...
Embodiment 3
[0036] The synthesis of embodiment 3L-carnosine
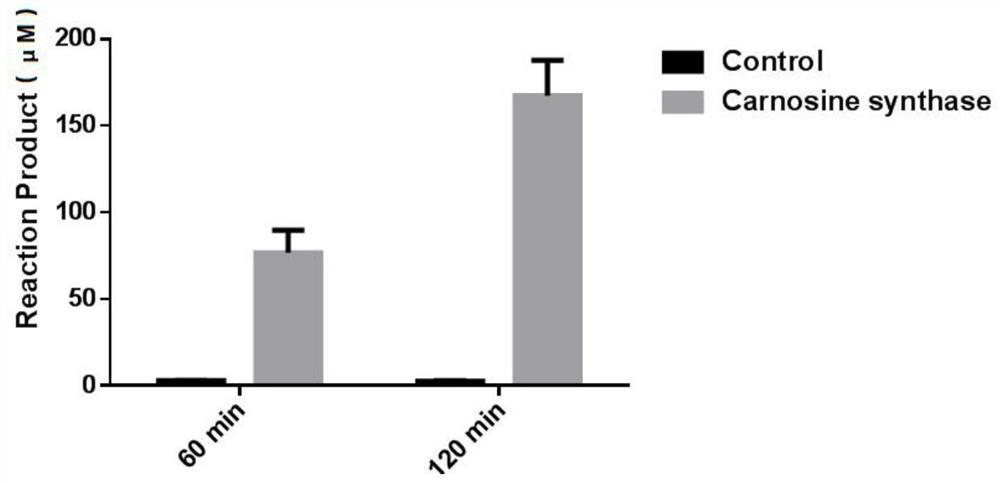
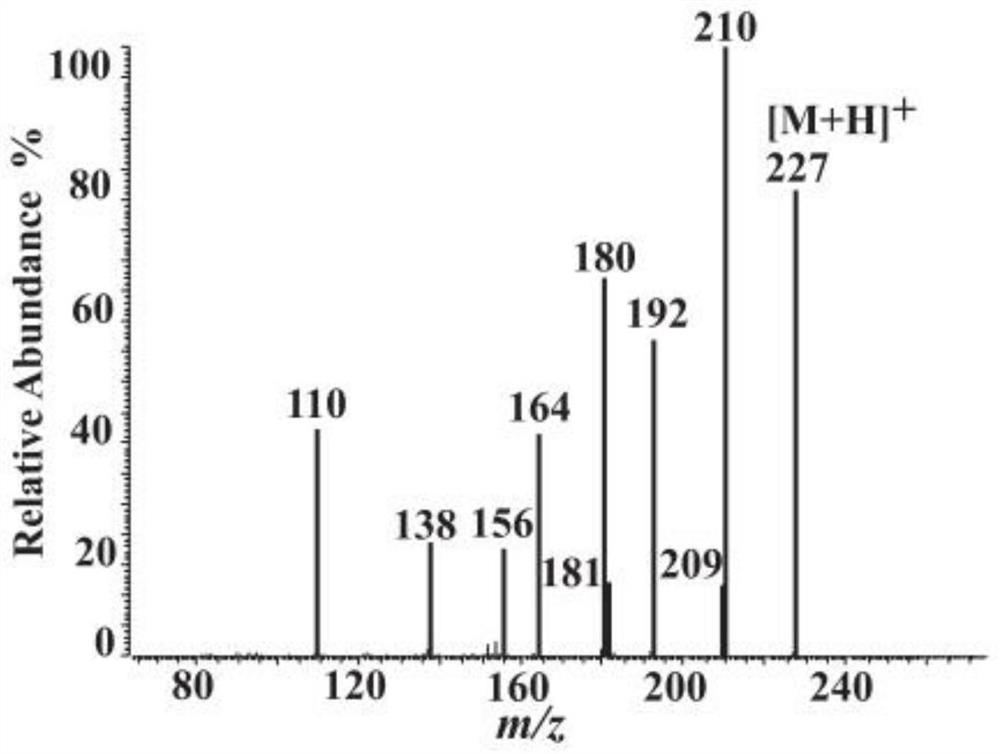
[0037]Using the 0.2ml solution (PBS containing 20mMGSH as a solvent) containing about 15μg Tridacna maxima ATPGD recombinant protein obtained in Example 2 and 1ml containing 0.5mM β-alanine, 0.5mM L-histidine and 0.5mM The PBS substrate solution of MgATP was incubated at 37°C for 60min and 120min; the control group was 0.2ml solution containing 15μg BSA protein (PBS containing 20mM GSH as solvent) and 1ml containing 0.5mM β-alanine, 0.5 A PBS substrate solution of mM L-histidine and 0.5 mM MgATP was mixed. Three biological repetitions were set up for each group, and then 200 μl of 30% (v / v) HCl was added to terminate the reaction, and macromolecules such as proteins were removed after filtration with a 3 kDa ultrafiltration membrane. Use HPLC to measure the concentration of the filtrate containing carnosine, and find that the reaction system containing the ATPGD recombinant protein of giant clam can produce 76.8 μ M and 167.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com