Naphthalene-indandione donor-acceptor compound, preparation method thereof and application of naphthalene-indandione donor-acceptor compound in lipid droplet washing-free fluorescent probe
A fluorescent probe and indenedione technology, applied in the field of biomedicine, can solve the problems of unsuitable fatty liver disease diagnosis, unstable chemical structure, low selectivity and sensitivity, and achieve excellent photostability and biocompatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0054] The third embodiment of the present invention provides a composition containing the above-mentioned naphthalene-indanedione donor-receptor compound or a pharmaceutically acceptable salt thereof.
[0055] The fourth embodiment of the present invention provides a preparation comprising the above-mentioned naphthalene-indanedione donor-receptor compound or a pharmaceutically acceptable salt or composition thereof and a pharmaceutically acceptable carrier.
[0056] The carrier of the present invention can be water, buffer solution (phosphate, etc.) and the like.
[0057] The fifth embodiment of the present invention provides an application of the above-mentioned naphthalene-indanedione donor-receptor compound, composition and / or preparation in a lipid droplet wash-free fluorescent probe.
[0058] The sixth embodiment of the present invention provides an application of the above-mentioned naphthalene-indanedione donor-receptor compound, composition and / or preparation in diag...
Embodiment 1
[0063] Embodiment 1: the synthesis of probe ANI
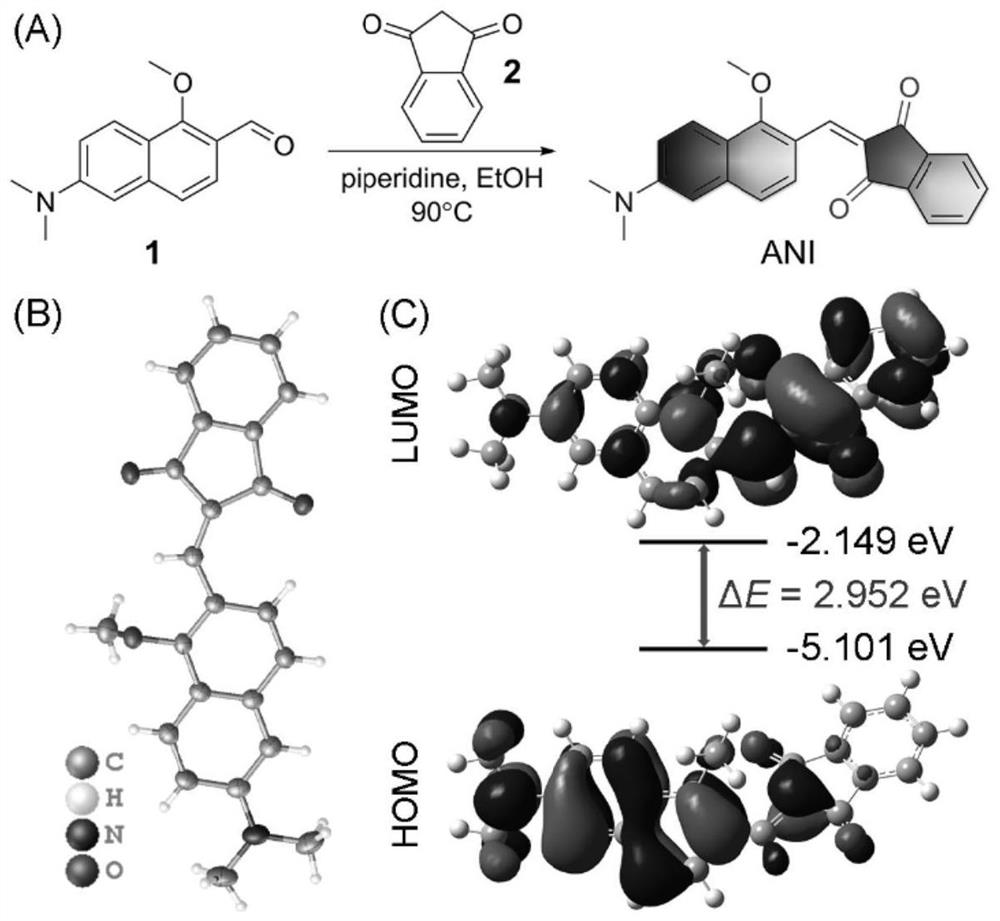
[0064] Compound 1 and compound 2 undergo a nucleophilic reaction to obtain the probe ANI( figure 1 A). Compound 1 (115mg, 0.5mmol), compound 2 (88mg, 0.6mmol) and a few drops of piperidine were refluxed at 90°C for 8h in absolute ethanol (10mL), cooled to room temperature, and the solvent was removed under reduced pressure. AcOEt (10:1, 6:1, to 1:1 by volume ratio) was used as the eluent, and the residue was purified by silica gel chromatography to obtain a green solid (mass 133 mg, yield 74%). 1 H NMR spectrum, 13 C NMR spectrum and high-resolution mass spectrometry were as Figure 9 , Figure 10 and Figure 11 shown. And X-ray single crystal diffraction proved the structure of ANI ( figure 1 B), the corresponding unit cell parameters and refined data are shown in Table 1.
[0065] The detailed characterization data are as follows: 1 H NMR (400MHz, CDCl 3 ),δ(ppm):9.02(d,J=9.0Hz,1H),8.52(s,1H),8.07(d,J=9.3Hz,1H),8.02...
Embodiment 2
[0068] Example 2: Basic photophysical properties
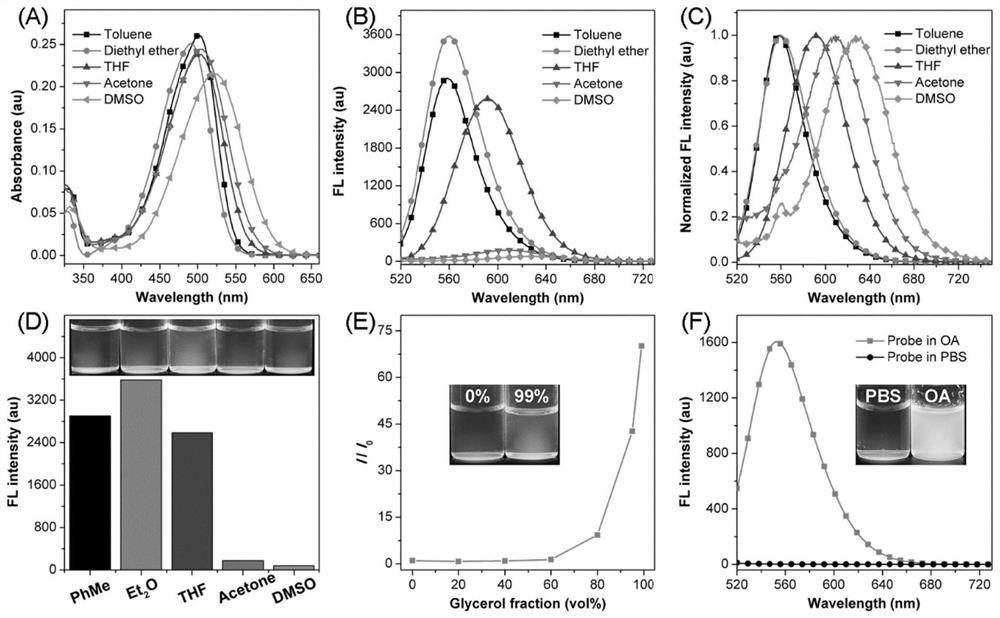
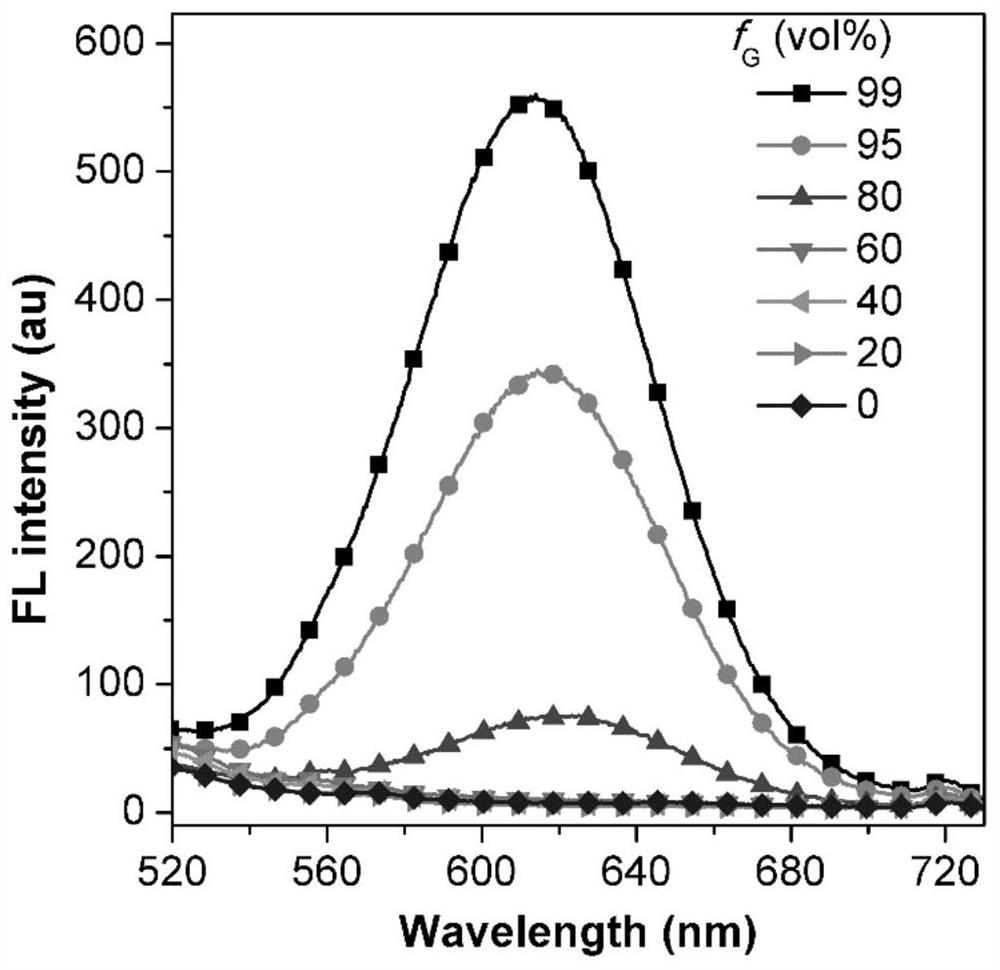
[0069] First, the HOMO and LUMO electron cloud distributions of the probe ANI were calculated by density functional theory ( figure 1 C), the electron cloud of HOMO is basically delocalized in the substituted naphthalene ring part, while the electron cloud of LUMO is mainly distributed in the 1,3-indanedione part. This apparent electron separation reveals a strong charge transfer effect and a narrow energy gap. Next, the detailed photophysical properties of ANI were tested. A test solution containing 5 μM of different polar solvents (toluene, ether, tetrahydrofuran, acetone, dimethyl sulfoxide, PBS) was prepared, and the above solution was tested with a UV-visible spectrophotometer and a fluorescence spectrometer for its absorption spectrum and fluorescence emission spectrum ( figure 2 and image 3 ). Maximum absorption peak (λ abs ) in the small range of 492-523nm, with increasing polarity from toluene to DMSO, λ abs ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com