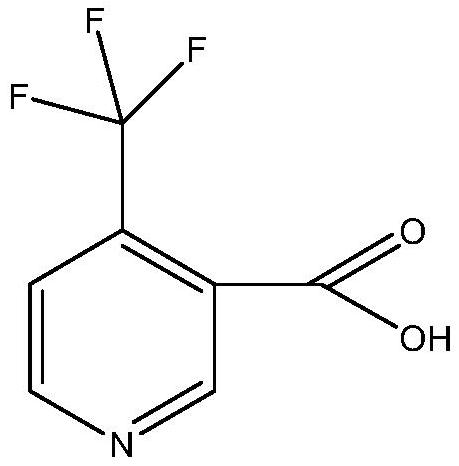
Synthesis method of 4-trifluoromethyl nicotinic acid
A technology of trifluoromethyl nicotinic acid and synthesis method, applied in directions such as organic chemistry, can solve the problems of difficult industrialized production, long process route, expensive raw materials, etc., and achieve easy industrialization, easy separation and purification, and easy control of reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
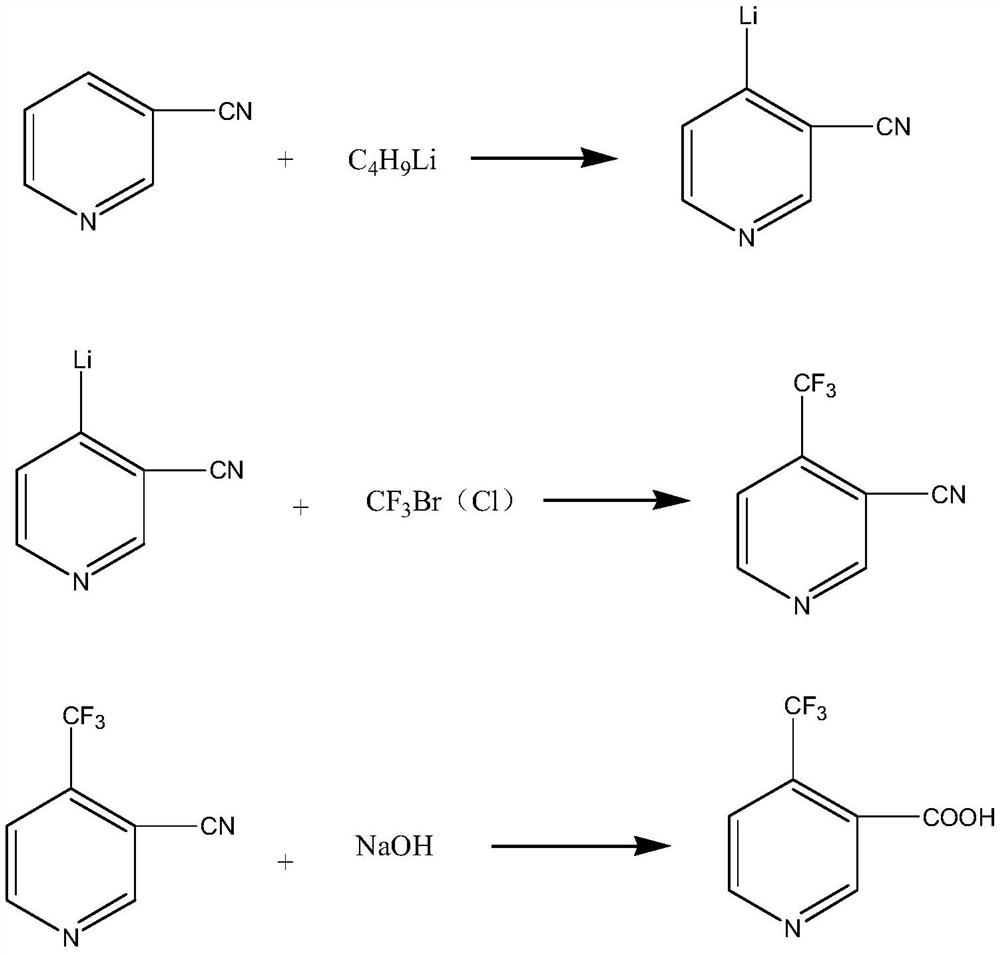
[0035] This embodiment provides a kind of synthetic method of 4-trifluoromethyl nicotinic acid, and this method comprises the following steps:
[0036] Step 1, add 3-cyanopyridine (104g, 1.0mol), tetramethylethylenediamine (174g, 1.5mol) and 600ml of tetrahydrofuran into a three-necked flask replaced with argon, and cool to -40 under the protection of argon. ℃, keep -40 ℃, add 2M butyllithium (600ml, 1.2mol), keep the reaction for 1.5h, and seal it for use.
[0037] Step 2: Cool the solution prepared in the previous step to -40°C, pass bromotrifluoromethane (200g, 1.3mol) into the solution, then react at -40°C for 2h, then raise the temperature to 0°C for 4h, and then use saturated Quenched with ammonium chloride solution, extracted with 1000ml of petroleum ether, dried over anhydrous magnesium sulfate, and distilled to obtain 141g of 4-trifluoromethyl-3-cyanopyridine.
[0038] Step 3: Add 100g of 4-trifluoromethyl-3-cyanopyridine, 300ml of water and 75g of sodium hydroxide i...
Embodiment 2
[0047] This embodiment provides a kind of synthetic method of 4-trifluoromethyl nicotinic acid, and this method comprises the following steps:
[0048] Step 1, add 3-cyanopyridine (10.4g, 0.1mol), tetramethylethylenediamine (174g, 1.5mol) and 800ml of petroleum ether into a three-necked flask replaced with argon, and cool to -40°C, keep at -40°C, add 2M butyllithium (600ml, 1.2mol), keep it warm for 1.5h, seal it for use.
[0049] Step 2, cool the solution prepared in the previous step to -40°C, pass chlorotrifluoromethane (140g, 1.35mol) into the solution, then react at -40°C for 2h, then raise the temperature to 0°C for 4h, and then use saturated The ammonium chloride solution was quenched, and the layers were separated. After drying with anhydrous magnesium sulfate, 120 g of 4-trifluoromethyl-3-cyanopyridine was obtained by distillation.
[0050] Step 3: Add 100g of 4-trifluoromethyl-3-cyanopyridine, 300ml of water and 75g of sodium hydroxide into a 1000ml three-neck flask...
Embodiment 3
[0054] This example provides a synthetic method for 4-trifluoromethylnicotinic acid. The synthetic method of this example is basically the same as that of Example 1, the difference being that the solvent used in step 1 of this example is Methyltetrahydrofuran.
[0055] The characterization results of this example are the same as Example 1.
[0056] The total yield of 4-trifluoromethylnicotinic acid synthesized based on 3-cyanopyridine is 70%, and the purity is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com