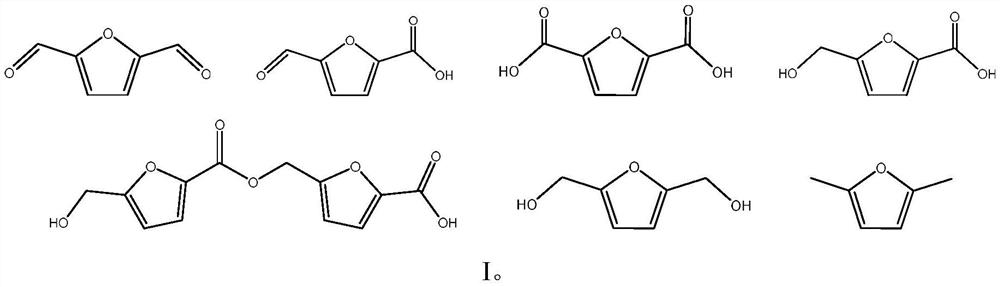
Method for one-step synthesis of 5-hydroxymethylfurfural and derivatives thereof by using microwave-microreactor to catalyze fructose
A technology of hydroxymethyl furfural and microreactor, applied in chemical instruments and methods, chemical/physical process, chemical/physical/physical chemical process, etc., can solve the problem of poor catalyst stability, low HMF selectivity, difficult separation and purification, etc. problem, to achieve the effect of less by-products, low energy consumption and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: The preparation process of wave-absorbing catalyst MOx / CNTs is as follows:
[0039] Modified carbon nanotubes were obtained by vapor deposition: 5 g of multi-walled carbon nanotubes were placed in a corundum crucible and placed in a tube furnace. Vacuumize the tube furnace and feed pure argon gas, the argon gas flow rate is 100mL / min, and the temperature is raised to 900 °C at a heating rate of 5-10 °C / min; then feed ammonia gas with a volume fraction of 5%, and keep 2h to obtain nitrogen-doped carbon nanotubes, and slowly lower to room temperature after heating.
[0040] Preparation of MOx / CNTs catalyst by impregnation method: 0.5 g nitrogen-doped carbon nanotubes were impregnated in 5 mL metal salt solution (50 mmol AgCl, CeCl 3 , CuCl 2 , MnCl 2 、CoCl 2 , FeCl 3 , ZrCl 4 dissolved in 5mL deionized water), ultrasonicated for 30min, stirred for 2h to fully disperse, washed with deionized water and ethanol, and vacuum-dried for 2h; then calcined the ...
Embodiment 2
[0041] Embodiment 2: Carried out fructose one-step synthesis HMF in microwave-microreactor
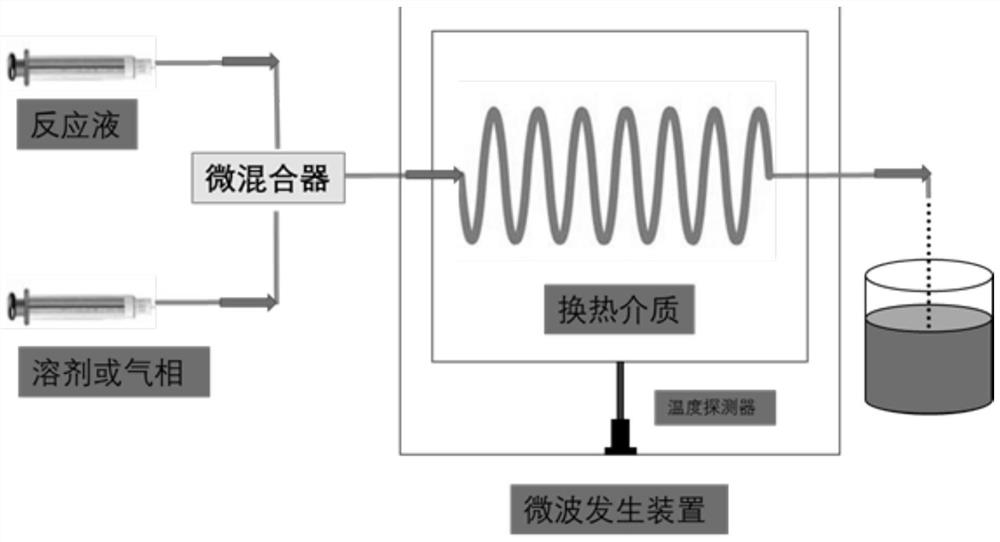
[0042] 6g fructose and 0.5g Co 3 o 4 / CNTs were dissolved in 50 mL deionized water and ultrasonicated for 30 minutes; in the flow mode, the suspension of fructose and wave-absorbing catalyst was used as one phase, the flow rate was set at 0.05 mL / min, and 4-methyl-2-pentanone was used as the other phase. phase, the flow rate is set to 0.5mL / min, the two phases are mixed by a micro-mixer for 20 minutes, and then passed into a U-shaped spiral glass pipe in the heating medium in the microwave reactor. After the temperature of the heating medium is stable, react at 90°C 10 minutes.
[0043] After the reaction ended, the resulting reaction solution took out the organic phase 4-methyl-2-pentanone, and the gained organic phase was diluted with ethanol to a certain number of times. It was qualitatively observed that the main product was HMF by high performance liquid chromatography, and the ...
Embodiment 3
[0044] Embodiment 3: Carried out fructose one-step synthesis FDCA in microwave-microreactor
[0045] Mix 6g fructose and 0.5g CeO 2 / CNTs were dissolved in 50 mL dimethyl sulfoxide and ultrasonicated for 30 minutes; in the flow mode, the suspension of fructose and wave-absorbing catalyst was used as one phase, the flow rate was set at 0.02 mL / min, and pure oxygen was used as the other phase, and the oxygen pressure 2bar, the flow rate is set to 0.1mL / min, the two phases are mixed by a micro-mixer for 20min, and then passed into the U-shaped spiral glass pipe in the heating medium in the microwave reactor. After the temperature of the heating medium is stabilized, the React for 20 minutes.
[0046] After the reaction, the obtained reaction solution was diluted with ethanol to a certain number of times, and the main product was qualitatively observed by high performance liquid chromatography to be FDCA. The yield of FDCA was measured to be 88.61%, and the selectivity was 90.42%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com