Flomoxef process impurity and preparation method thereof
A process impurity, the technology of flumoxef sodium, which is applied in the field of medicine, can solve the problems of no patent report of impurities, many steps in the synthesis process of flumoxef sodium, and poor stability of intermediates, etc., and achieve the effect of guaranteeing safe clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
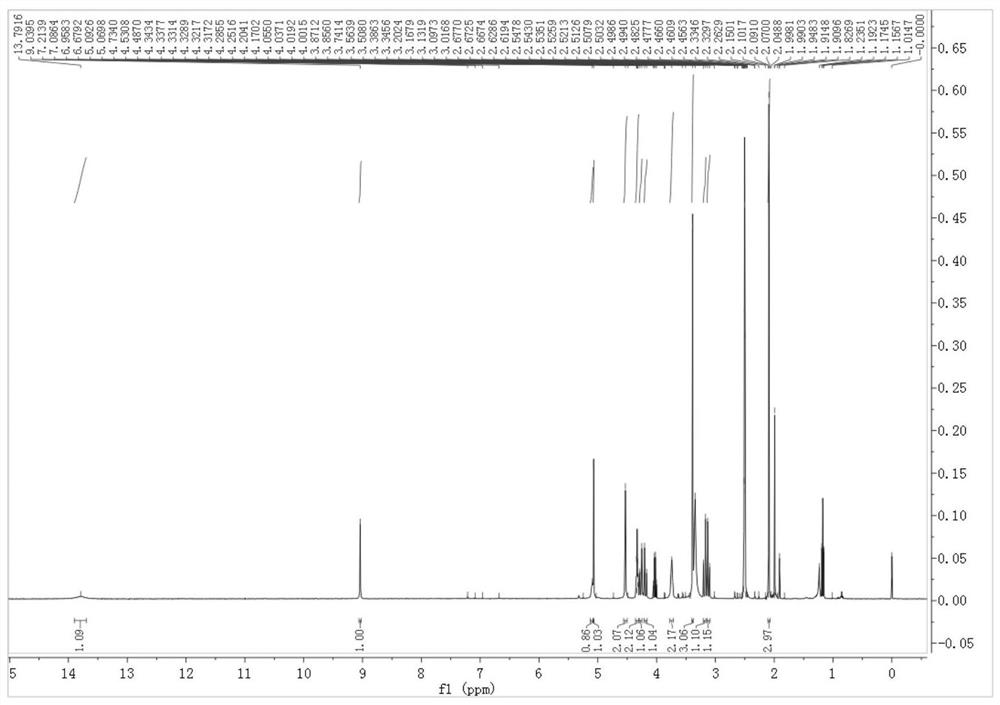
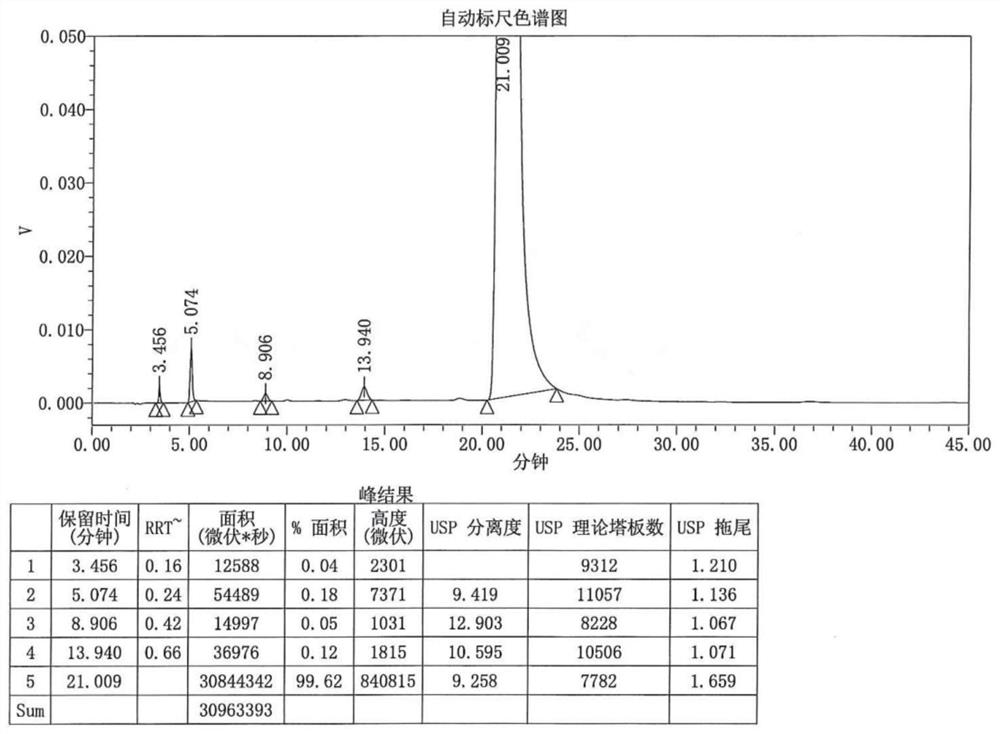
[0045] 1. The specific HPLC detection method is as follows:
[0046]Use octadecylsilane bonded silica gel as filler (C18 4.6mm×250mm, 5μm), and phosphate buffer solution (take 6.94g potassium dihydrogen phosphate, 3.22g disodium hydrogen phosphate dodecahydrate and 1.60g tetrahydrogen phosphate n-Butylammonium bromide (dissolved in 1000ml of water)-methanol (75:25) as the mobile phase, the column temperature is 25°C, the detection wavelength is 246nm, the flow rate is 1.0ml per minute, and the injection volume is 5μl.
[0047] 2. The concrete synthetic method of this impurity is as follows:
[0048]
[0049] Dissolve 1g (9.42mmol) of methylthioacetic acid in 10ml of dichloromethane, add 2.98g (37.68mmol) of pyridine, under the protection of nitrogen, stir and cool down to -15~-20°C, add flumoxefin dropwise to the reaction solution Sodium intermediate 2.02g (4.71mmol) (6R,7R)-3-(chloromethyl)-7-amino-7-methoxy-8-oxo-5-oxa-1-azabicyclo[4.2 .0] Oct-2-ene-2-carboxylic acid be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com