Tedizolid phosphate, preparation method of intermediate of tedizolid phosphate and injection
A technology of tedizolid phosphate and intermediates, applied in the field of preparation methods and injections of tedizolid phosphate and intermediates thereof, can solve the problems of unsuitability for industrialized production, harsh reaction conditions, low production costs and the like, and achieve improved selectivity. and catalytic, low production cost, easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1Pd
[0035] The preparation of preparation example 1Pd series catalyst
[0036] S1. Preparation of silica magnetic microspheres: Add 10g of γ-aminopropyltrimethoxysilane and 4g of ferrous chloride into water, add dropwise 25wt% ammonia solution, adjust the pH to 9, heat to 80°C, and Ultrasonic while dropping, the power is 1000W, after 2 hours of reaction, magnet separation, washing, to obtain silica magnetic microspheres;
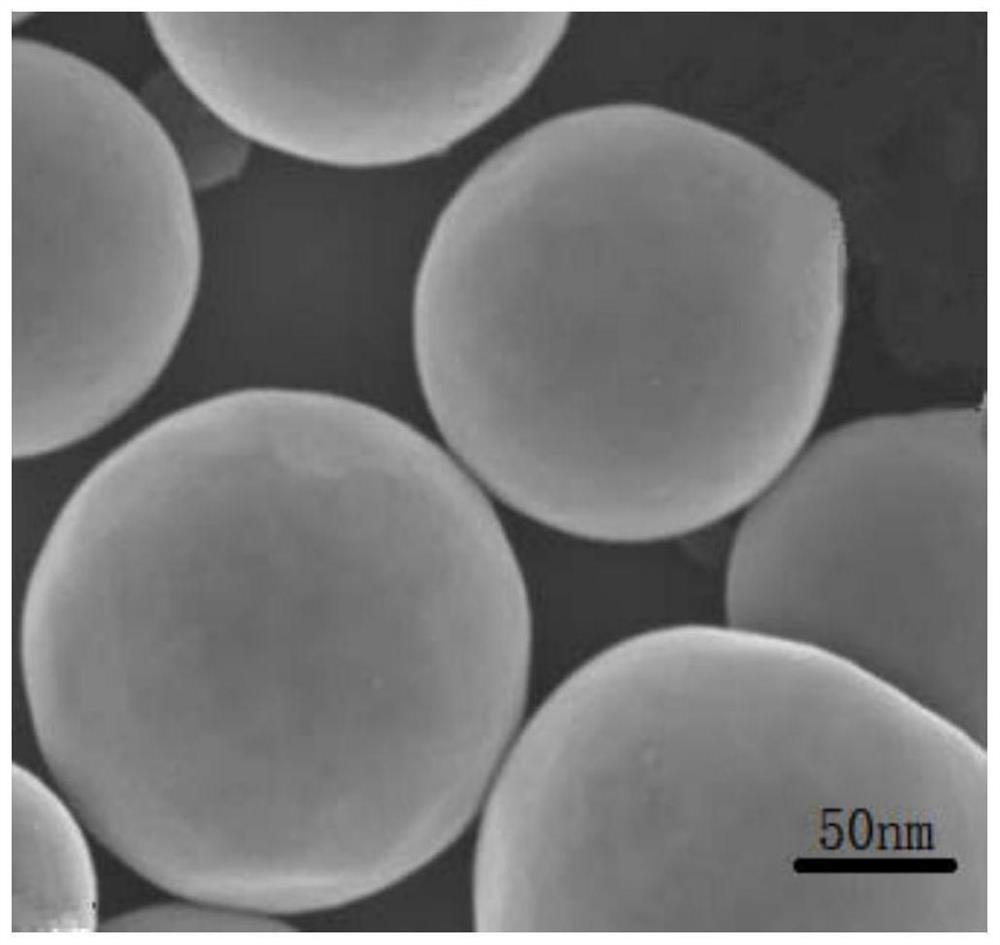
[0037] S2. Preparation of Pd-loaded silica magnetic microspheres: Disperse 1 g of silica magnetic microspheres in water, disperse evenly by ultrasonic, add 0.82 g of PdCl 2 , heated to 50° C., reacted for 1 h, separated by a magnet, and washed to obtain a Pd-based catalyst, and the amount of Pd-containing substances per g of the Pd-based catalyst was 0.001 mol. figure 1 It is a SEM image of the Pd-based catalyst, and it can be seen from the image that the particle size of the microspheres is between 100-500nm.
Embodiment 1
[0039] synthetic route:
[0040]
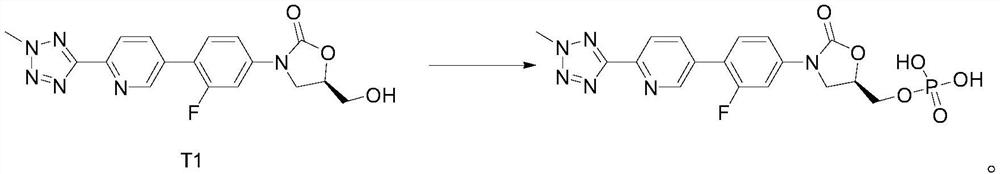
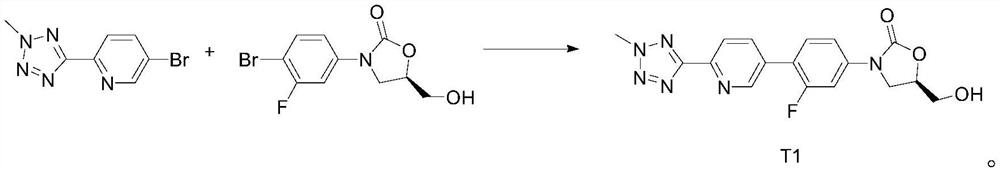
[0041] Step 1, the synthesis of intermediate T1:
[0042] Add 2-methyl-5-(5-bromopyridin-2-yl)tetrazolium 20.00g, biboronic acid pinacol ester 27.51g, the Pd-based catalyst prepared in Preparation Example 1 to a dry 500mL four-necked flask 3g, KOAc 24.53g, 1,4-dioxane 300mL, stirring, N 2 Replace three times. Replacement completed, N 2 Under protection, the temperature was raised to 90° C., and the reaction was incubated for 2 hours. The reaction was monitored by TLC (2-methyl-5-(5-bromopyridin-2-yl)tetrazolium spots disappeared). Under nitrogen protection, 150 mL of purified water, 22.96 g of (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one, K 2 CO 3 32.82g stirred and reacted for 2h. After the reaction is complete, turn off the heating, lower the temperature naturally, stir and crystallize, filter for 18 hours, and rinse the filter cake with 100 mL of 1,4-dioxane: water = 1:1 mixture and 100 mL of purified water. ...
Embodiment 2
[0049] Step 1, the synthesis of intermediate T1:
[0050] Add 40.00 g of 2-methyl-5-(5-bromopyridin-2-yl)tetrazolium, 55.2 g of pinacol ester of diboronic acid, and the Pd-based catalyst prepared in Preparation Example 1 into a dry 1000 mL four-necked flask 5g, KOAc 49.06g, 1,4-dioxane 600mL, stirred, replaced with Ar gas three times. After the replacement, the temperature was raised to 90° C. under the protection of Ar gas, and the reaction was incubated for 3 hours. TLC monitored the completion of the reaction (2-methyl-5-(5-bromopyridin-2-yl)tetrazolium spots disappeared). Under the protection of Ar gas, 300 mL of purified water and 45.92 g of (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one were added to the above reaction mixture 、K 2 CO 3 65.64g stirred and reacted for 2h. After the reaction is complete, turn off the heating, lower the temperature naturally, stir and crystallize, filter for 20 hours, and wash the filter cake with 200 mL of 1,4-dioxane...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com