Xylanase Scxyn5 as well as coding gene and application thereof
A xylanase and gene technology, applied in the field of genetic engineering, can solve problems such as different physical and chemical properties and biological functions, and achieve the effects of reducing costs, reducing use, and preventing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Cloning of the gene encoding xylanase Scxyn5 of embodiment 1
[0036] Schizophyllum commune was fermented and cultured with corn bran fiber as a carbon source, and the mRNA was isolated from the bacteria for transcriptome sequencing analysis; the Scxyn5 coding gene (Xyn5-F: 5′-CCG GAATTC CTCCCCAAGCGTCAGACCACT-3′; Xyn5-R: 5′-CGCTA GCGGCCGC TGCACCCAAG CCGCTCAC-3'), and constructed the recombinant vector Scxyn5-pET28a.
Embodiment 2
[0037] The construction of embodiment 2 xylanase recombinant vectors
[0038]Using the xylanase Scxyn5-pET28a derived from S.commune sp.DB1 as a template, the PCR reaction was used to clone the Scxyn5 fragment of the xylanase gene. The primers and reaction conditions were as follows:
[0039] Scxyn5-F: 5′-CCG GAATTC CTCCCC AAGCGTCAGCCACT-3′;
[0040] Scxyn5-R: 5′-CGCTA GCGGCCGC TGCACCCAAG CCGCTCAC-3';
[0041] The amplified products were digested by EcoRI and NotI, respectively, and directly ligated with the cut pPIC9k plasmid, transformed into TransI-T1 competent cells, and the transformants were picked and sequenced for verification. The correct transformant verified by sequencing was the recombinant yeast expression plasmid pPIC9K-Scxyn5.
Embodiment 3
[0042] Example 3 Construction of the Pichia pastoris engineering bacteria recombinantly expressing the xylanase gene
[0043] The recombinant expression vector pPIC9K-Scxyn5 was linearized with endonuclease SacI and transformed into Pichia pastoris GS115 to obtain recombinant yeast strain GS115 / pPIC9K-Scxyn5.
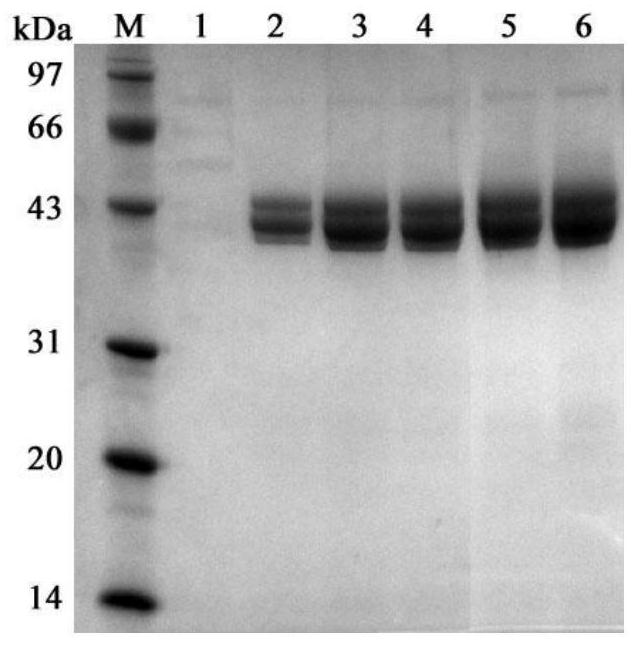
[0044] Pick the positive transformant and transfer it to a 50mL Erlenmeyer flask containing 5mL of BMGY medium, place it at 30°C, and culture it on a shaker at 230rpm for 36h; centrifuge the fermentation broth at 3000g for 5min, discard the supernatant and use 5mL of BMMY medium to precipitate the bacteria Resuspended, then placed at 30°C, 230rpm shaker induction culture for 72h. Such as figure 1 As shown, the supernatant of the fermentation broth was used for enzyme activity detection and SDS-PAGE electrophoresis detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com