Human recombinant arginase I producing strain and construction method thereof
A technology of arginase and construction method, applied in microorganism-based methods, biochemical equipment and methods, recombinant DNA technology and other directions, can solve problems such as recombinant arginase I and other problems that have never been seen, and achieve convenient purification treatment, The effect of fast growth and increased activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Plasmid Transformation
[0039] Using the pHT304 plasmid (purchased from Youbao Biology) as the basic plasmid, it was transformed as follows:
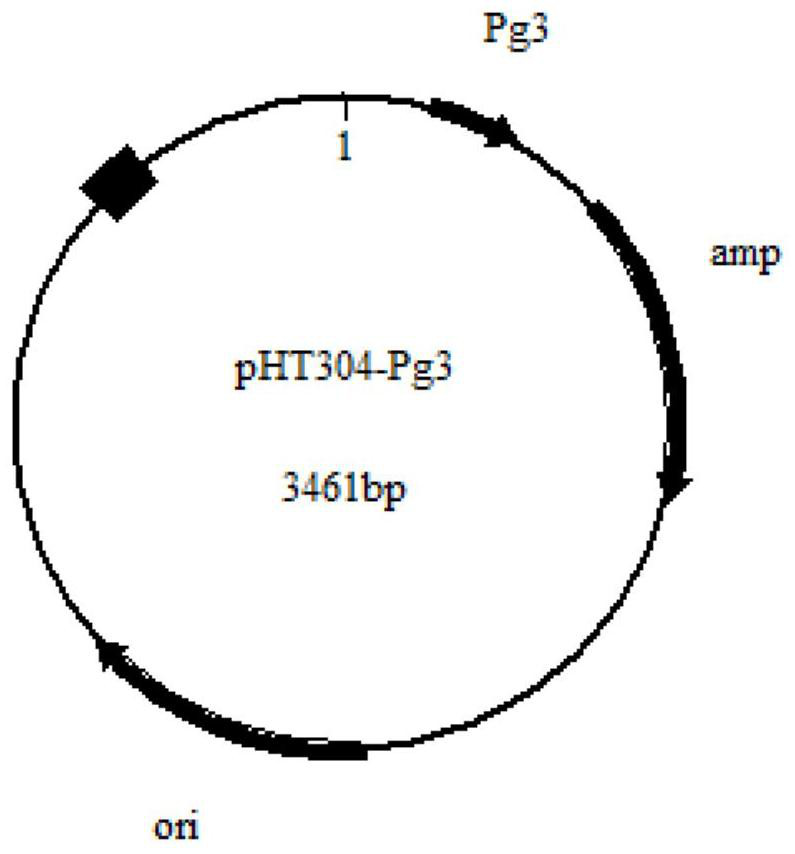
[0040] The pHT304 plasmid was digested with NdeI and HincII, and then the Pg3 promoter (sequence shown in SEQ ID NO.1) was integrated into the pHT304 plasmid after double digestion to construct the plasmid pHT304-Pg3 ( figure 1 ).
[0041] The constructed plasmid pHT304-Pg3 is resistant to single ampicillin, has a lactose operon, and its nucleotide sequence is shown in SEQ ID NO.2.
[0042] The purpose of the present invention to transform the pHT304 plasmid is: one is to reduce the length of the vector and improve its expression stability in Bacillus subtilis; Therefore, after integrating the Pg3 promoter, the bacterial strain grows fast in the early stage, and the time required to enter the stable phase is short, thereby shortening the expression time of human recombinant arginase I, and improving the expression t...
Embodiment 2
[0043] Example 2: Optimization and transformation of arg1 gene
[0044] The inventor obtained the amino acid sequence of human arginase I from the existing database as shown in SEQ ID NO.3; the nucleotide sequence of its encoding gene arg1 is shown in SEQ ID NO.4.
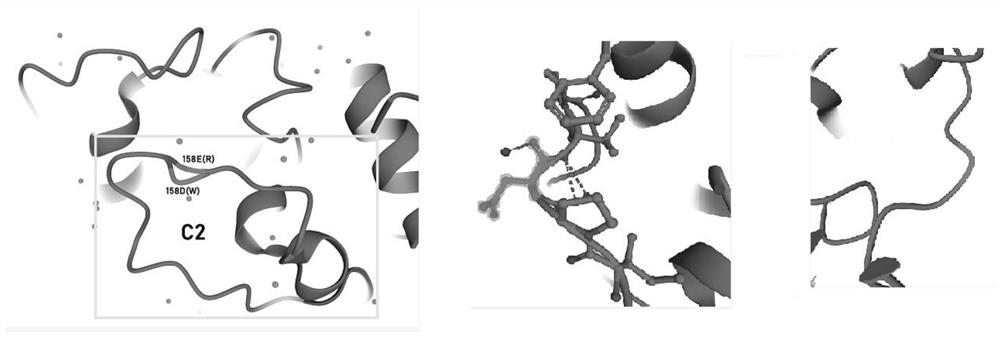
[0045] In order to further improve the activity of human recombinant arginase I, the inventors used molecular dynamics simulation to optimize the structure of human recombinant arginase I, and the 158th asparagus of human recombinant arginase I Amino acid is mutated into glutamic acid, and the amino acid sequence of the mutated human recombinant arginase I is shown in SEQ ID NO.5.
[0046] Schematic diagram of the three-dimensional structure of human arginase I figure 2 As shown, aspartic acid (D) at the 158th position protrudes outwards. After being mutated to glutamic acid (E), it does not protrude outwards, and the angle between P and V is also reduced, so the overall structure Also more compact.
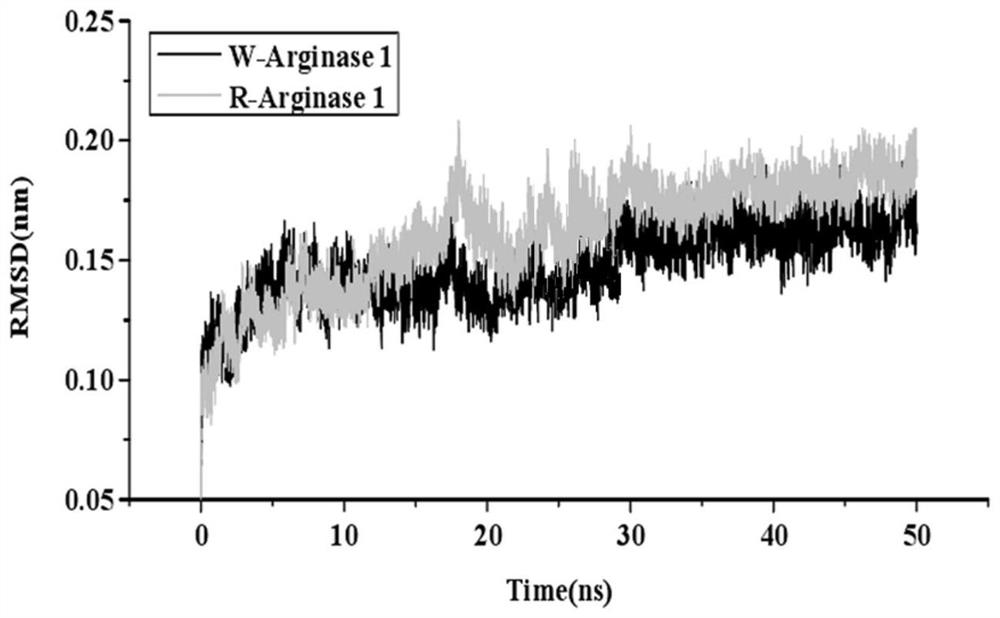
[0047] Afte...
Embodiment 3
[0055] Embodiment 3: Construction of recombinant expression vector
[0056] The transformed plasmid pHT304-Pg3 in Example 1 was treated with SphI and SacI double-enzyme digestion and double-enzyme digestion, and then the arg1 gene (arg1Δ158, whose nucleotide sequence was as SEQ ID NO. 7) was integrated into the plasmid pHT304-Pg3 after the double enzyme digestion treatment to obtain the recombinant expression vector (pHT304-Pg3-arg1); the structural diagram of the recombinant expression vector is shown in Figure 7 shown.
[0057] The constructed recombinant expression vector was verified by electrophoresis, and the results were as follows: Figure 8 shown. The results showed that the arg1 gene (shown in SEQID NO.7) had been successfully integrated into the plasmid pHT304-Pg3. After sequencing verification, the nucleotide sequence of the constructed recombinant expression vector (pHT304-Pg3-arg1) is shown in SEQ ID NO.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com