Chitosan oligosaccharide enteric capsule as well as preparation method and application thereof
A technology of enteric-coated capsules and chitosan oligosaccharides, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve problems such as reduction, precipitation, and stratification of chitosan oligosaccharides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
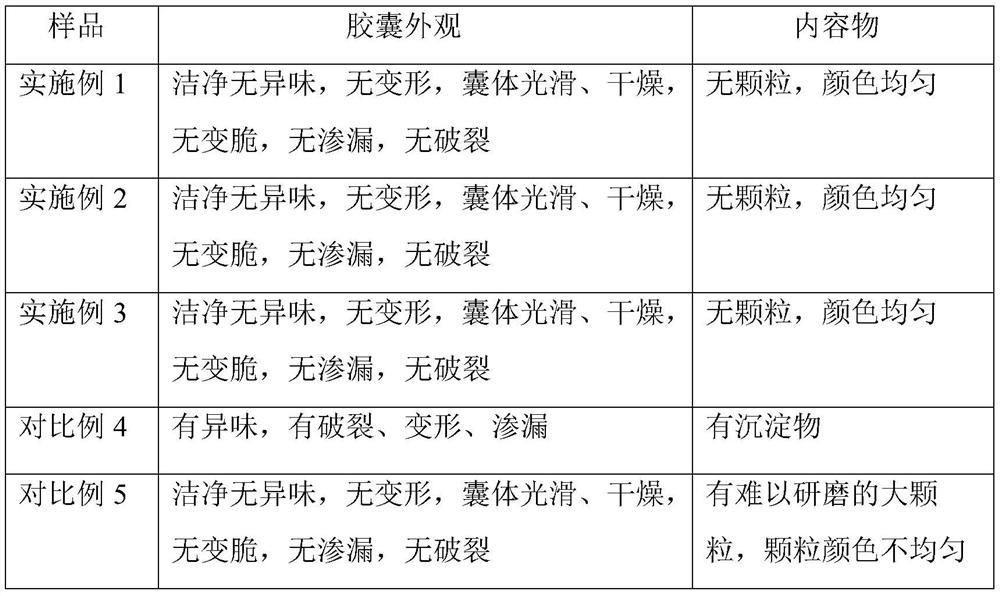
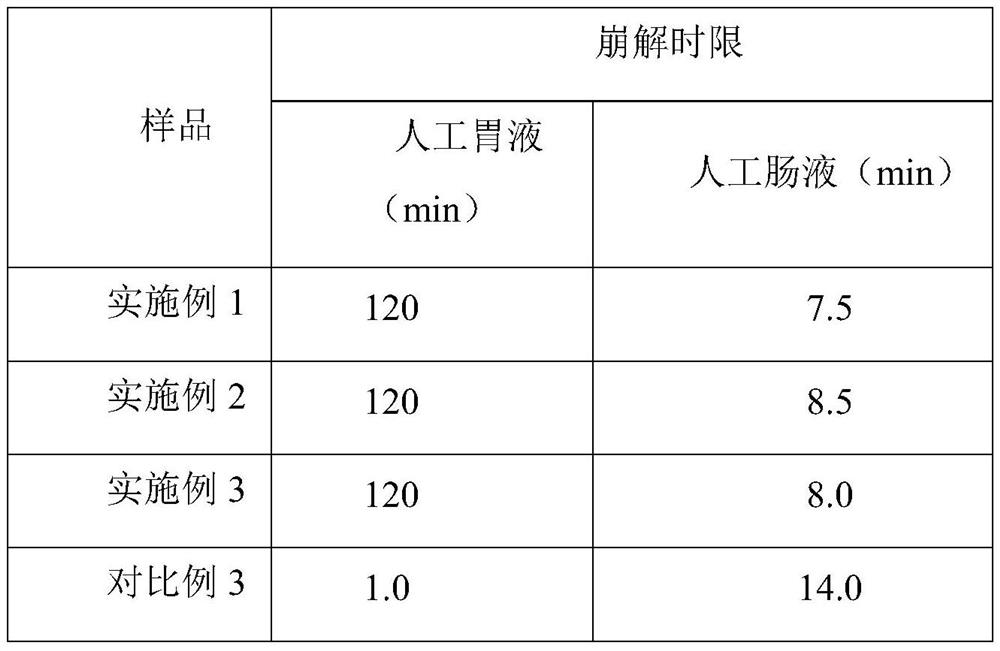
Embodiment 1
[0026] Embodiment 1, chitosan oligosaccharide enteric-coated capsule of the present invention and its preparation
[0027] Recipe: Oligochitosan 10g, Eudragit L100 40g, HPMC10g, Drcaps capsule shell
[0028] making process:
[0029] S1. Dissolution: Dissolve Eudragit L100 in water and let it swell for 0.5 hours to obtain Eudragit L100 solution; dissolve HPMC in water and let it stand for 0.5 hours to obtain HPMC solution; completely dissolve chitosan oligosaccharide in water to obtain chitosan oligosaccharide solution ;
[0030] S2. Stirring: Slowly add the chitosan oligosaccharide solution into the Eudragit L100 solution while stirring, then add the HPMC solution, and stir vigorously with a magnetic stirrer for 0.5h to obtain a mixed medicinal solution;
[0031] S3. Ultrasonic treatment: put the mixed medicinal solution into an ultrasonic instrument, and perform ultrasonic treatment at the highest frequency for 0.5 h at 37°C;
[0032] S4. Freeze-drying: The ultrasonically ...
Embodiment 2
[0034] Embodiment 2, oligochitosaccharide enteric-coated capsule of the present invention and its preparation
[0035] Recipe: Chitooligosaccharide 20g, Eudragit L100 80g, HPMC 20g, Drcaps capsule shell
[0036] making process:
[0037] S1. Dissolution: Dissolve Eudragit L100 in water and let it swell for 0.5 hours to obtain Eudragit L100 solution; dissolve HPMC in water and let it stand for 0.5 hours to obtain HPMC solution; completely dissolve chitosan oligosaccharide in water to obtain chitosan oligosaccharide solution ;
[0038] S2. Stirring: Slowly add the chitosan oligosaccharide solution into the Eudragit L100 solution while stirring, then add the HPMC solution, and stir vigorously with a magnetic stirrer for 0.5h to obtain a mixed medicinal solution;
[0039] S3. Ultrasonic treatment: put the mixed drug into an ultrasonic instrument, and perform ultrasonic treatment at the highest frequency at 37°C for 0.5h;
[0040] S4. Freeze-drying: The ultrasonically treated mixed...
Embodiment 3
[0042] Embodiment 3, oligochitosaccharide enteric-coated capsule of the present invention and its preparation
[0043] Recipe: Chitooligosaccharide 30g, Eudragit L100 120g, HPMC 30g, Drcaps capsule shell
[0044] making process:
[0045] S1. Dissolution: Dissolve Eudragit L100 in water and let it swell for 0.5 hours to obtain Eudragit L100 solution; dissolve HPMC in water and let it stand for 0.5 hours to obtain HPMC solution; completely dissolve chitosan oligosaccharide in water to obtain chitosan oligosaccharide solution ;
[0046] S2. Stirring: Slowly add the chitosan oligosaccharide solution into the Eudragit L100 solution while stirring, then add the HPMC solution, and stir vigorously with a magnetic stirrer for 0.5h to obtain a mixed medicinal solution;
[0047] S3. Ultrasonic treatment: put the mixed medicinal solution into an ultrasonic instrument, and perform ultrasonic treatment at the highest frequency for 0.5 h at 37°C;
[0048] S4. Freeze-drying: The ultrasonic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com