Green synthesis method of tetravalent manganese ion activated fluoride red luminescent material
A technology of red luminescence and green synthesis, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor devices, etc., can solve the problems of unfavorable mass industrial production, insufficient synthesis conditions, long synthesis cycle, etc., and achieve the convenience of mass industry The effect of chemical preparation, large-scale production and fast synthesis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
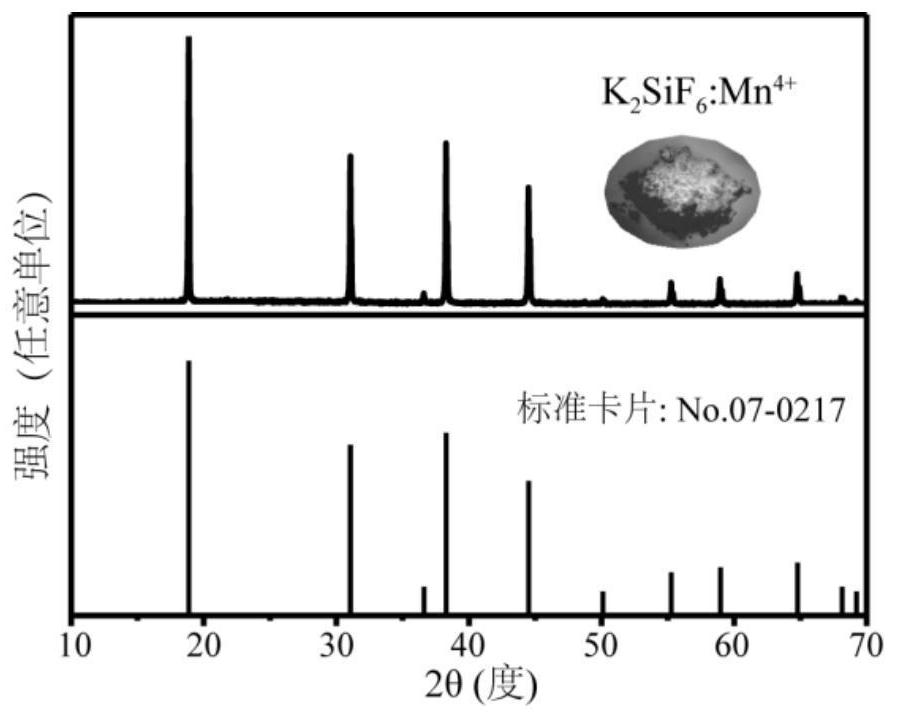
[0029] At room temperature, weigh potassium hexafluoromanganate (K 2 MnF 6 )0.0148g, completely dissolved in fluosilicic acid (H 2SiF 6 , 30wt%) solution to obtain solution 1, the molar concentration of potassium hexafluoromanganate in solution 1 is 0.025mol / L, take potassium fluoride (KF) 0.3486g and dissolve in deionized water completely to obtain solution 2, in solution 2 The molar concentration of potassium fluoride is 0.6mol / L, solution 1 and solution 2 are mixed and stirred for 5 minutes to obtain solution 3, the mol ratio of potassium fluoride and potassium hexafluoromanganate in solution 3 is 100.17:1, solution 3 The precipitate was obtained by centrifugation, washed 3 times with ethanol, and then dried at 75°C for 2 hours to obtain the final product red luminescent material K 2 Si 0.99 mn 0.01 f 6 .
[0030] Detect the obtained red luminescent material, such as figure 1 , Figure 5 and Figure 6 shown. Such as figure 1 As shown, the prepared sample is a pu...
Embodiment 2
[0032] At room temperature, weigh potassium hexafluoromanganate (K 2 MnF 6 )0.0445g, completely dissolved in fluosilicic acid (H 2 SiF 6 , 30wt%) solution to obtain solution 1, the molar concentration of potassium hexafluoromanganate in solution 1 is 0.076mol / L, and potassium hydrogen fluoride (KHF 2 ) 2.3430g is completely dissolved in deionized water to obtain solution 2, the molar concentration of potassium bifluoride in solution 2 is 3mol / L, solution 1 and solution 2 are mixed and stirred for 10 minutes to obtain solution 3, potassium bifluoride and six The molar ratio of potassium fluoromanganate is 166.58:1. The solution in the above steps is centrifuged to obtain a precipitate, washed 3 times with ethanol, and then dried at 75°C for 2 hours to obtain the final product red luminescent material K 2 Si 0.97 mn 0.03 f 6 .
Embodiment 3
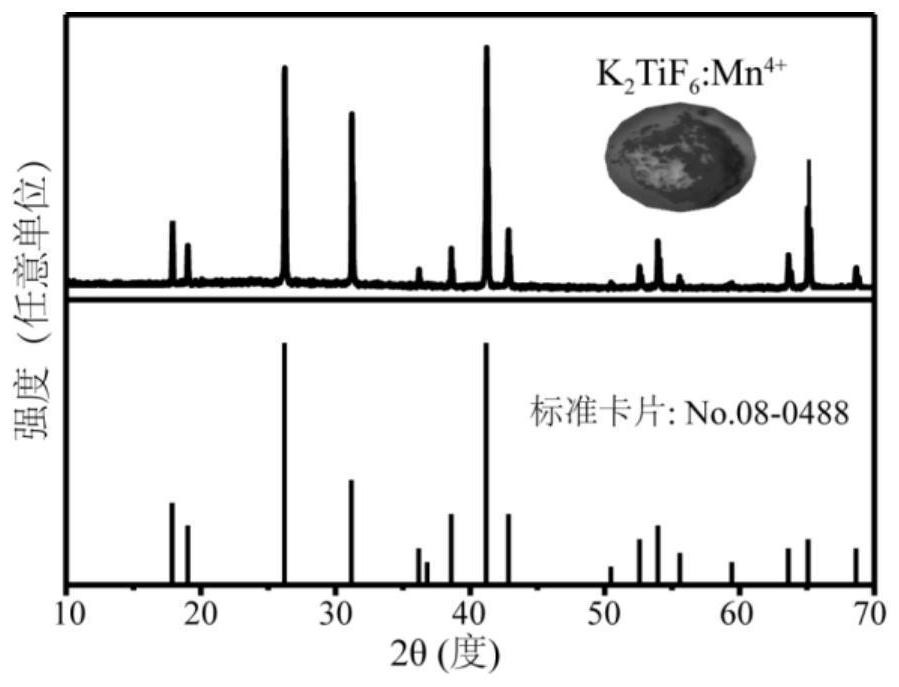
[0034] At room temperature, weigh potassium hexafluoromanganate (K 2 MnF 6 )0.0148g, completely dissolved in fluorotitanic acid (H 2 TiF 6 , 50wt%) solution to obtain solution 1, the molar concentration of potassium hexafluoromanganate in solution 1 is 0.051mol / L, and potassium bifluoride (KHF 2 ) 0.9372g, be completely dissolved in deionized water to obtain solution 2, the molar concentration of potassium bifluoride in solution 2 is 1.2mol / L, solution 1 and solution 2 are mixed and stirred for 10 minutes to obtain solution 3, potassium bifluoride in solution 3 The molar ratio to potassium hexafluoromanganate is 200.35:1. Centrifuge the solution in the above steps to obtain a precipitate, wash with ethanol three times, and then dry at 75°C for 2 hours to obtain the final product red luminescent material K 2 Ti 0.99 mn 0.01 f 6 .
[0035] Detect the obtained red luminescent material, such as figure 2 As shown, the prepared samples were pure phase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com