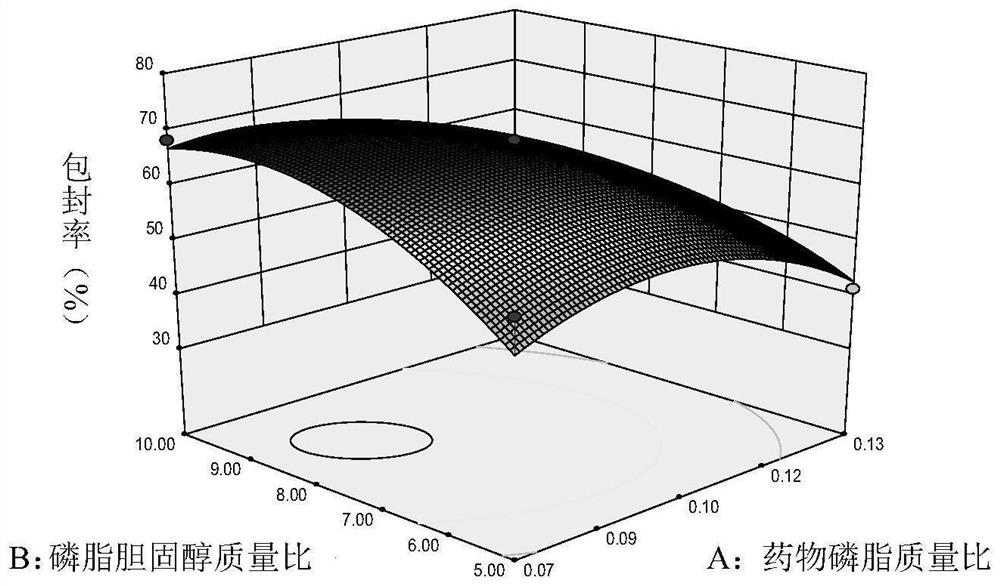
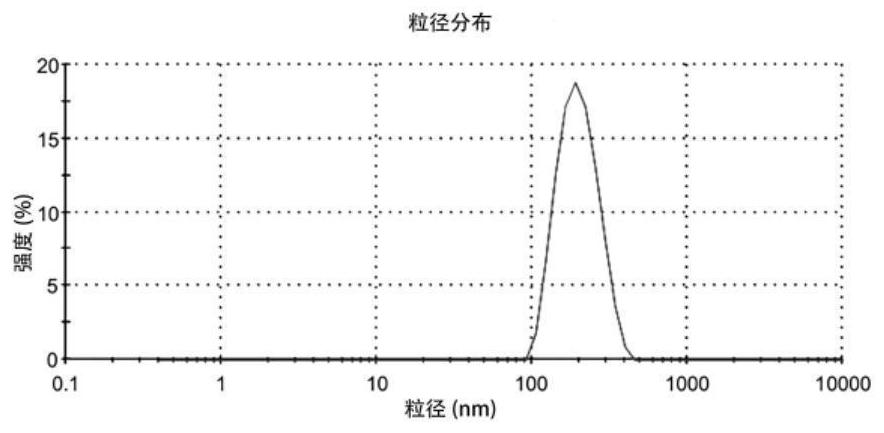
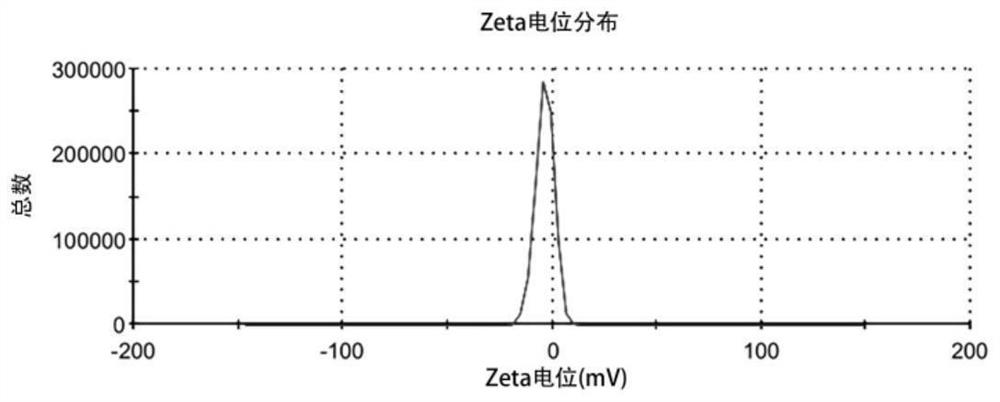
Ophthalmic cetirizine hydrochloride liposome, in-situ gel and preparation method of ophthalmic cetirizine hydrochloride liposome
A technology of cetirizine hydrochloride and ophthalmic liposomes, which is applied in the field of pharmaceutical preparations, can solve the problems of short residence time, poor compliance of semi-solid dosage forms, and difficult acceptance by patients, so as to delay drug release and improve bioavailability degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] Prescription and ratio: cetirizine hydrochloride 28.5mg, egg yolk lecithin 356.3mg, cholesterol 41.8mg, sodium chloride: 13mg, ethyl p-hydroxybenzoate: 10mg, ultrapure water to 10mL.
[0170] Preparation method: Weigh the prescribed amount of egg yolk lecithin and cholesterol, dissolve in 5mL ethanol, stir and ultrasonically dissolve as the oil phase; add 8mL of 59.39mg / mL ammonium sulfate solution to another beaker, keep the temperature in a water bath at 50°C, as Water phase: Use a disposable 5mL syringe to slowly inject the oil phase below the liquid level of the water phase, stir magnetically (1200 rpm) while adding, continue to stir at 50°C for 50min to evaporate ethanol, and then rotate at 50°C and 200rpm Remove ethanol by evaporation; then ultrasonically smooth the inner wall of the round bottom flask, put it into a pre-treated dialysis bag, place it in 500mL 0.9% NaCl solution for dialysis for 24 hours (change the dialysate every 2 hours for the first 6 hours, an...
Embodiment 2
[0252] The best prescription (10mL) of cetirizine hydrochloride lipothermal gel containing 0.298%: cetirizine hydrochloride: 29.8mg, egg yolk lecithin: 372.5mg, cholesterol: 43.67mg, F127: 2.55g, F68 : 0.6g, sodium chloride: 13mg, ethyl p-hydroxybenzoate: 10mg and ultrapure water to 10mL.
[0253] Weigh the prescribed amount of egg yolk lecithin and cholesterol and dissolve in 5mL of ethanol, stir and ultrasonically dissolve as the oil phase; add 8mL of 59.39mg / mL ammonium sulfate solution to another beaker and keep the temperature in a water bath at 50°C as the water phase; Use a disposable 5mL syringe to slowly inject the oil phase below the liquid level of the water phase, stir magnetically (1200 rpm) while adding, continue stirring at 50°C for 50 minutes to evaporate the ethanol, and then remove the ethanol by rotary evaporation at 50°C and 200rpm Then ultrasonically smooth the inner wall of the round-bottomed flask, put it into a pre-treated dialysis bag, place it in 500m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com