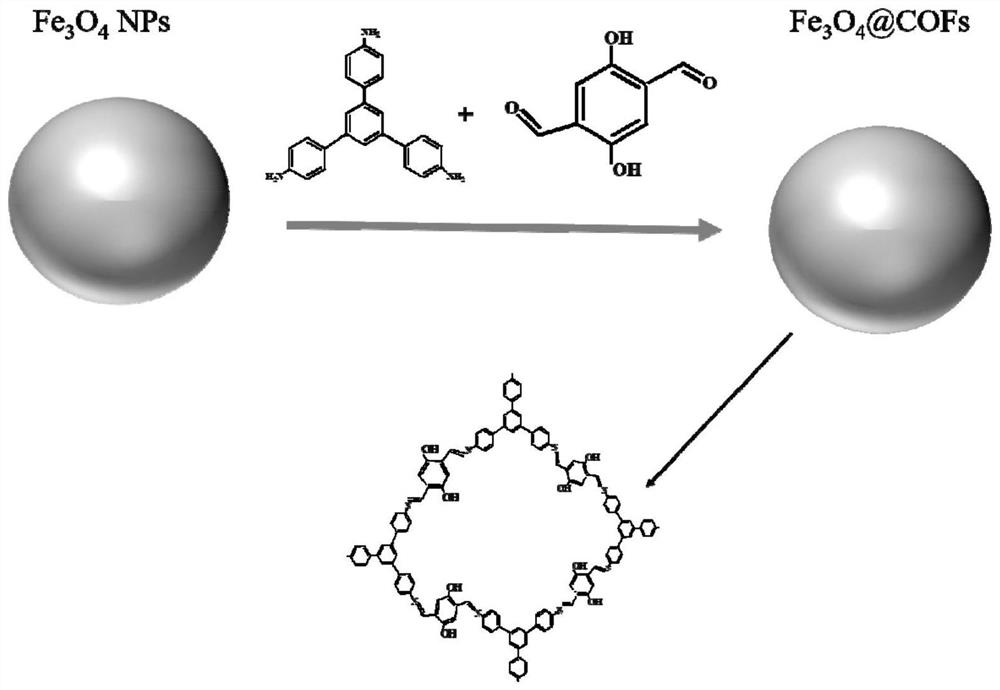
Magnetic covalent organic framework nano material, preparation method and application
A covalent organic framework and nanomaterial technology, applied in the field of magnetic covalent organic framework nanomaterials and preparation, can solve problems such as human health risks, achieve excellent magnetic separation characteristics, simple and convenient operation, and high adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of a magnetic covalent organic framework nanomaterial disclosed by the present invention comprises the following steps:
[0037] Step 1, preparation of magnetic Fe 3 o 4 nanoparticles;
[0038] The specific preparation method of the present invention is preferably: FeCl 3 、6H 2 O, Na 3 Cit, 2H 2 O. Dissolve NaAc in ethylene glycol and stir for 20-50 minutes. Among them, FeCl 3 、6H 2 O, Na 3 Cit, 2H 2 The mass ratio of O and NaAc is 2~6:0.5~2:3~7; the resulting mixed solution is placed in an autoclave and reacted at 150°C~200°C for 8~12h, and the obtained product is washed and dried to obtain magnetic Fe 3 o 4 nanoparticles.
[0039] Step 2, the magnetic Fe 3 o 4 Nanoparticles dispersed in ethanol to obtain Fe 3 o 4 Dispersion, preferably, magnetic Fe 3 o 4 The mass of nanoparticles and the volume ratio of absolute ethanol is 0.1~1g:5~50mL; 2,5-dihydroxy terephthalaldehyde is dissolved in ethanol, preferably, 2,5-dihydroxy terephthal...
Embodiment 1
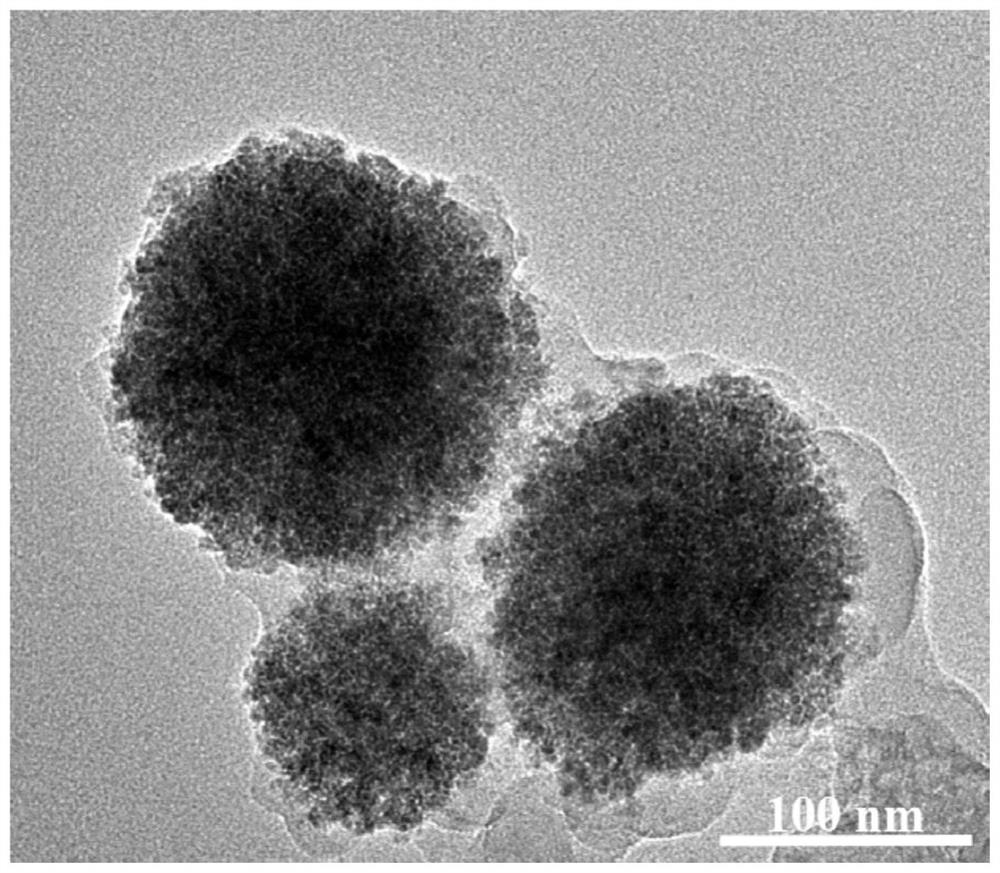
[0053] This embodiment discloses the preparation of a magnetic covalent organic framework nanomaterial, specifically:
[0054] Step 1, Magnetic Fe 3 o 4 Preparation of nanoparticles:
[0055] The raw materials are ferric chloride hexahydrate (FeCl 3 、6H 2 O) 3.4g, dehydrated sodium citrate (Na 3 Cit, 2H 2 O) 1.0 g and sodium acetate (NaAc) 6.0 g. The organic solvent used is ethylene glycol 100mL. FeCl 3 、6H 2 O, Na 3 Cit, 2H 2 O. NaAc was dissolved in ethylene glycol, stirred by ultrasonic to form a homogeneous yellow solution, then transferred to a stainless steel autoclave lined with polytetrafluoroethylene, and reacted at 200°C for 12 hours. The material was taken out, washed repeatedly with ethanol and water, dried at 25°C, and set aside.
[0056] Step 2, functionalization of COFs materials: take 0.5g of magnetic Fe prepared in step 1 3 o 4 Particles were ultrasonically dispersed in 25mL ethanol, 0.07g 2,5-dihydroxyterephthalaldehyde was ultrasonically dissol...
Embodiment 2
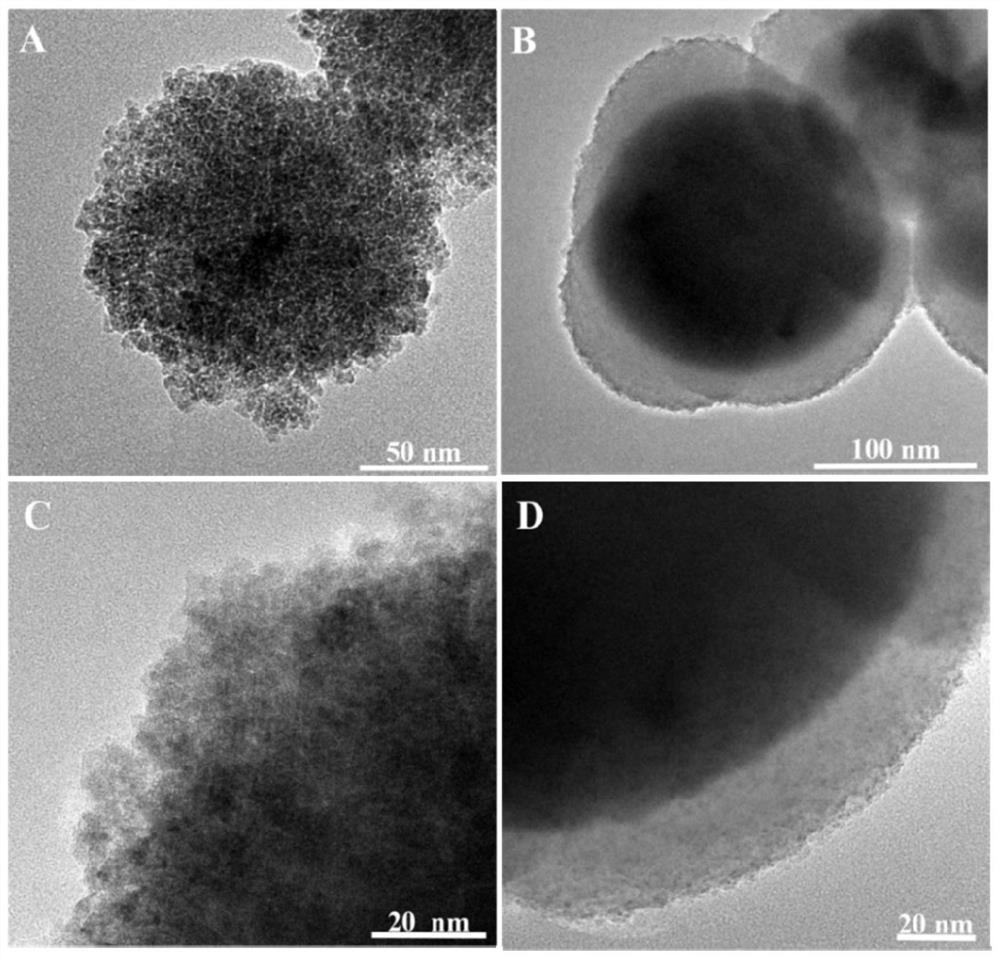
[0062] The difference between this embodiment and embodiment 1 is: in step 2, Fe 3 o 4 0.15g and 0.03g of 2,5-dihydroxyterephthalaldehyde.
[0063]The magnetic nanocomposite material Fe prepared in this embodiment 3 o 4 The topography of @COFs is similar to that of Example 1, and will not be given separately here.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Particle size range | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com