Preparation method of levosalbutamol hydrochloride
A technology of levosalbutamol hydrochloride and potassium carbonate, which is applied in the field of preparation of levosalbutamol hydrochloride, can solve the problems of low yield, heavy metals, pollution, etc., and achieve the effect of good yield, low equipment requirements and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
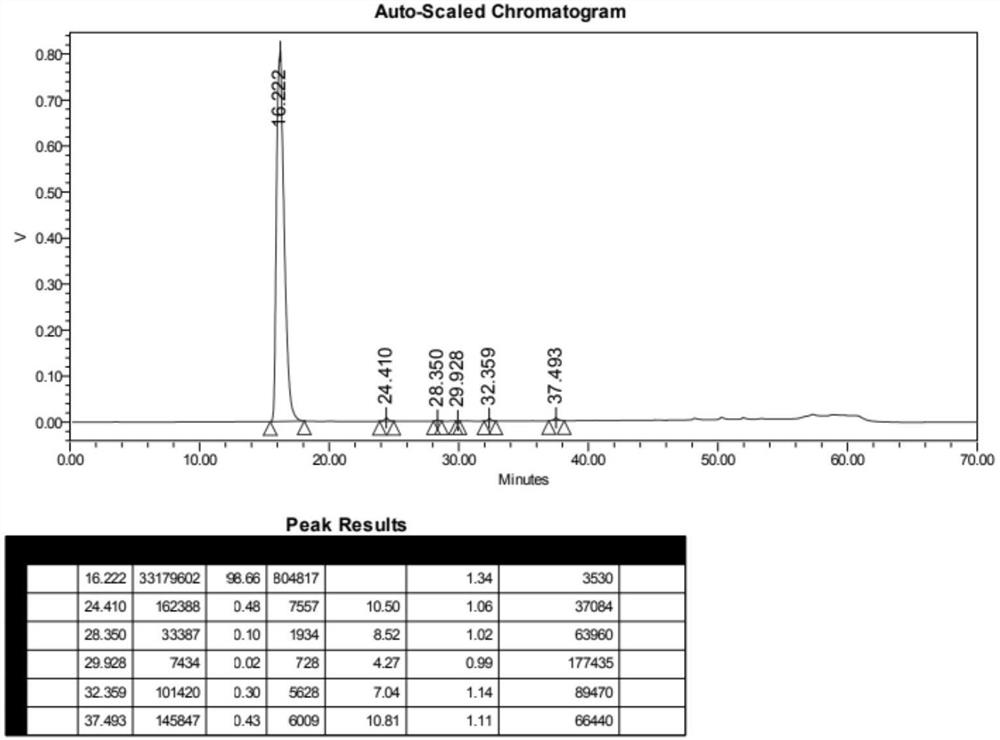
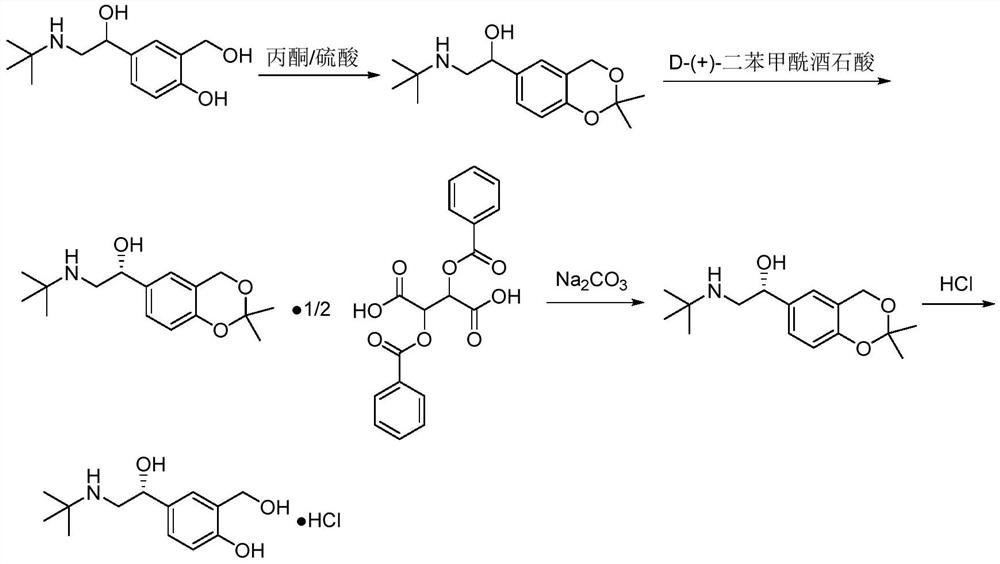
Image
Examples
Embodiment 1
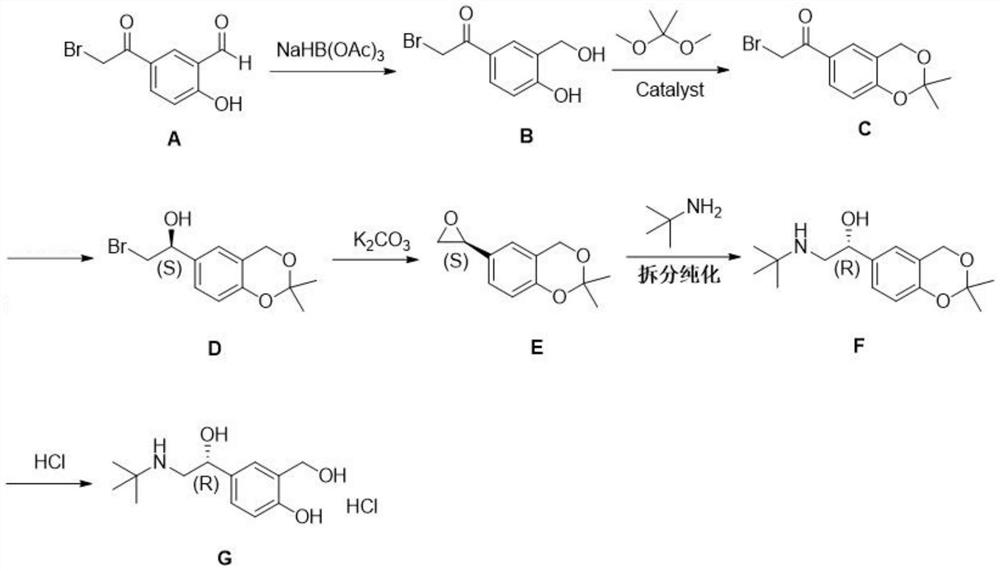
[0052] Synthesis of Example 1 Compound B (5-(2-bromoacetyl)-2-hydroxybenzyl alcohol)
[0053]
[0054] (1) Weigh 200.53g of compound A (5-(2-bromoacetyl)-2-hydroxybenzaldehyde), add 2.0L tetrahydrofuran and stir to dissolve, cool down to 15°C; weigh 263.51g sodium triacetoxyborohydride , added to the reaction system in batches, the temperature is controlled at 20±5°C, the feeding time is 120min, and the reaction is 60min at 20°C;
[0055] (2) HPLC and TLC monitor until the reaction is complete, add 2.0L of purified water, quench at room temperature for 60min, concentrate below 40°C to remove most of the tetrahydrofuran, add 1.6L of ethyl acetate to stir and extract, let stand to separate layers, and then use 0.4 Extract with L ethyl acetate once, combine the organic phases, wash once with 1.0L purified water; add an appropriate amount of anhydrous sodium sulfate to remove water;
[0056] (3) Filter, wash the filter residue with a small amount of ethyl acetate, collect the ...
Embodiment 2
[0058] Synthesis of Example 2 Compound C (6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin)
[0059]
[0060] (1) Weigh 150.06g compound B (5-(2-bromoacetyl)-2-hydroxybenzyl alcohol), add 1.5L dichloromethane, stir and disperse at room temperature, add 1.16g p-toluenesulfonic acid monohydrate; weigh 128.87g of 2,2-dimethoxypropane was added dropwise into the reaction system, and reacted at room temperature for 2h;
[0061] (2) Monitor by HPLC and TLC until the reaction is complete, add 0.75L of purified water, adjust the pH to 7-8 with saturated potassium bicarbonate solution, leave to separate layers, collect the lower organic phase, and wash the organic phase with 0.75L of purified water for 1 times; add anhydrous sodium sulfate to the organic phase to remove water;
[0062] (3) Filtrate, wash the filter residue with a small amount of dichloromethane, and concentrate under reduced pressure to obtain 174.01 g of oil, which is compound C (6-bromoacetyl-2,2-dimethyl-4H-benzo[...
Embodiment 3
[0064] Example 3 Synthesis of compound D (6-(2-bromo-1-(S)-ethanol)-2,2-dimethyl-4H-benzo[1,3]dioxin)
[0065]
[0066] (1) Weigh 9.21g of (1R,2S)-(+)-cis-1-amino-2-indanol, add 1.5L of anhydrous tetrahydrofuran, replace the nitrogen, cool down to 5±5°C, under the protection of nitrogen, Slowly add 150.31g of N,N-diethylaniline borane (DEANB) dropwise, after the addition is complete, keep warm at 5±5°C for 30min;
[0067] (2) Compound C (6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin) was dissolved by adding 1.5L of anhydrous tetrahydrofuran and stirring, under nitrogen protection, dropwise Into the reaction system of (1) above, the dropwise addition time is 180min, and the temperature is maintained at 5±5°C; the dropwise addition is completed, and the reaction is 30min;
[0068] (3) HPLC and TLC monitor until the compound C (6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin) is consumed, and the intermediate compound D is generated;
[0069] (4) 200ml of acetone was added d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com