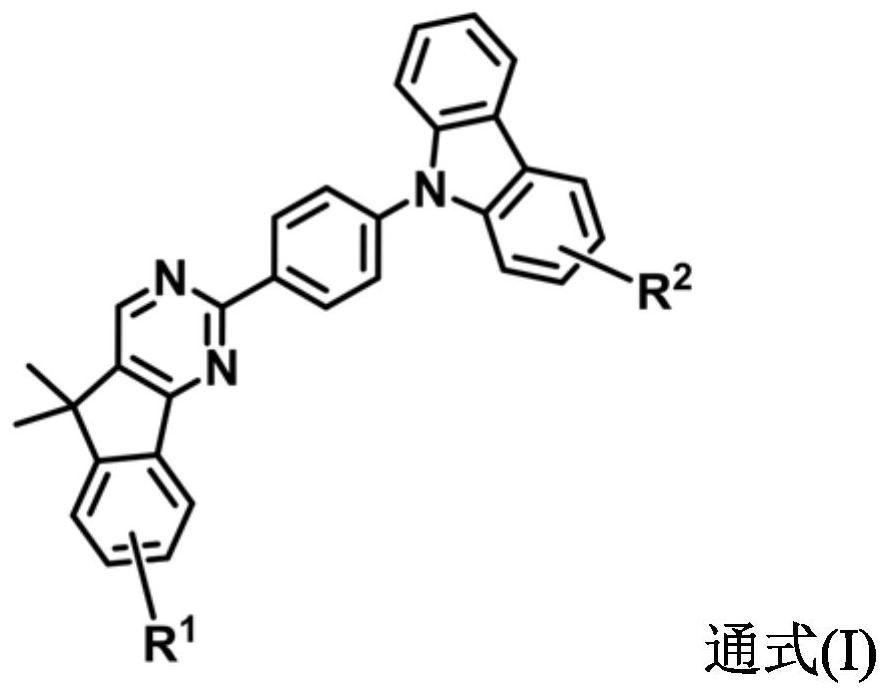
2, 4-diazafluorene derivative and electronic device
A technology of diazafluorene and electronic devices, which is applied in the field of electronic devices of organic electroluminescent devices, and can solve problems such as reduction in transmission performance, differences, and unbalanced mobility of electrons and holes in devices, and achieve high luminous efficiency , simple preparation method and low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
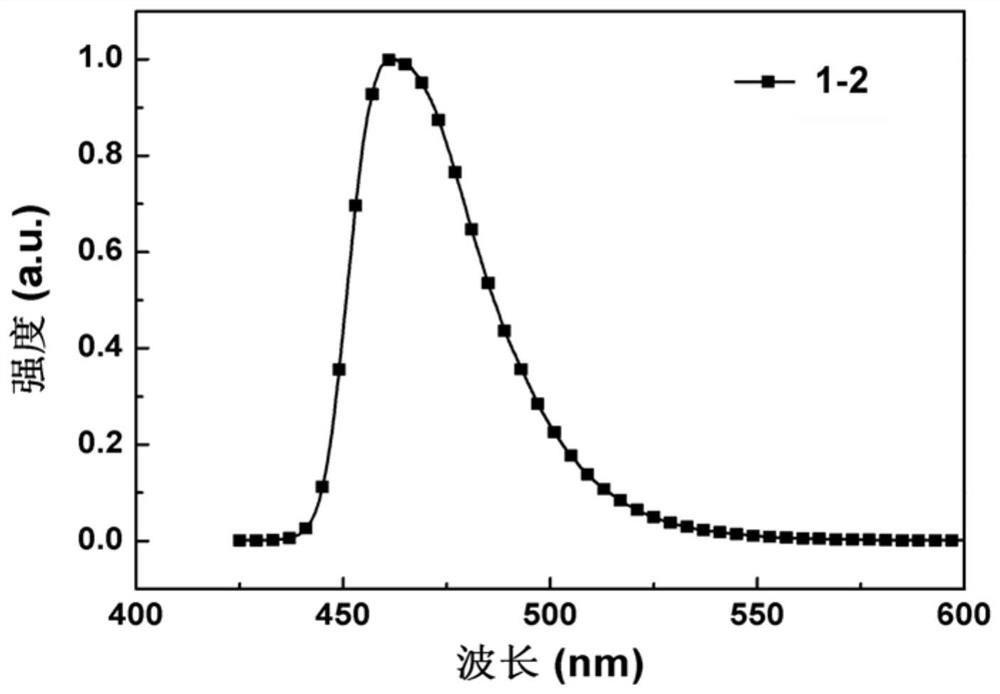
[0045] Embodiment 1: the synthesis of compound 1-2
[0046] The synthetic route of intermediate M1 is as follows:
[0047]
[0048] Add 1-indanone (2.64 g, 20 mmol) and N,N-dimethylformamide dimethyl acetal (5 mL, 37.6 mmol) into a 50 mL single-necked flask, and react under reflux for 6 hours. After the reaction was completed, cooled, and the solid was collected by suction filtration, and washed with a small amount of petroleum ether to obtain 3.52 g of an orange-red solid, with a yield of 94%. The crude product was directly used in the next reaction without further purification. MS(EI): m / z: 187.14[M + ]. Anal.calcd for C 12 h 13 NO (%): C76.98, H 7.00, N 7.48; found: C76.96, H 7.02, N 7.46.
[0049] Sodium methoxide (2.03g, 37.6mmol), 4-bromobenzamidine hydrochloride (8.87g, 37.6mmol) and 50mL of absolute ethanol were sequentially added into a dry and clean 100mL single-necked flask. Under constant stirring, 20 mL of anhydrous ethanol solution of the product from t...
Embodiment 2
[0056] Embodiment 2: the synthesis of compound 2-3
[0057] The synthetic route of compound 2-3 is as follows:
[0058]
[0059] Under nitrogen protection, intermediate M2 (1.76g, 5mmol), 5,7-dihydro-5-phenylindolo[2,3-B]carbazole (1.7g, 5.2 mmol), palladium acetate (11mg, 0.05mmol), tri-tert-butylphosphine tetrafluoroborate (29mg, 0.1mmol), sodium tert-butoxide (960mg, 10mmol) and 120mL toluene, refluxed and stirred for 12 hours. After the reaction was complete, the solvent was evaporated, the residue was dissolved with 200 mL of dichloromethane, washed with water, the organic layer was separated, the aqueous layer was extracted twice with 15 mL of dichloromethane, and the organic layers were combined. After distilling off the solvent, the residue was separated by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 1.9 g of white solid was obtained, the yield was 63%. MS(EI): m / z: 602.32[M + ]. Anal.calcdfor...
Embodiment 3
[0060] Embodiment 3: the synthesis of compound 3-3
[0061] The synthetic route of compound 3-3 is as follows:
[0062]
[0063] Under nitrogen protection, intermediate M2 (1.76g, 5mmol), 5,7-dihydro-7,7-dimethyl-indeno[2,1-B]carbazole (1.5 g, 5.2mmol), palladium acetate (11mg, 0.05mmol), tri-tert-butylphosphine tetrafluoroborate (29mg, 0.1mmol), sodium tert-butoxide (960mg, 10mmol) and 120mL toluene, reflux and stir for 12 hours . After the reaction was complete, the solvent was evaporated, the residue was dissolved with 200 mL of dichloromethane, washed with water, the organic layer was separated, the aqueous layer was extracted twice with 15 mL of dichloromethane, and the organic layers were combined. After distilling off the solvent, the residue was separated by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 2.0 g of white solid was obtained, yield 72%. MS (EI): m / z: 553.35 [M + ]. Anal.calcd for C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com