Affinity chromatography medium based on recombinant protein A as well as preparation method and application of affinity chromatography medium
A technology of recombinant protein and chromatographic medium, which is applied in the biological field to achieve the effects of reducing steric hindrance, improving alkali resistance and high dynamic load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1 Gene synthesis and plasmid preparation
[0068] 1. Gene synthesis
[0069] The coding gene of recombinant protein A (SEQ ID NO.7 and SEQ ID NO.8) was assembled from 50 double-stranded DNAs with 25-30 base pairs, as described in the reference (Krebs, et al. Synthesis of a gene forsensory rhodopsin I and its functional expression in Halobacterium halobium.Proc Natl Acad.Sci US A.1993,90(8):3486-3490), that is, 25 pairs of complementary DNA oligonucleotides were prepared by a DNA synthesizer, and then After phosphorylation, DNA oligonucleotide annealing, and ligation of 25 double-stranded DNAs, the entire synthetic gene is generated. The gene sequence encoding recombinant protein A of SEQ ID NO.7 is shown in SEQ ID NO.9, and the gene sequence encoding recombinant protein A of SEQ ID NO.8 is shown in SEQ ID NO.10.
[0070] 2. Preparation of expression vector
[0071] Insert the coding genes SEQ ID NO.9 and SEQ ID NO.10 of the synthetic recombinant protein A ...
Embodiment 2
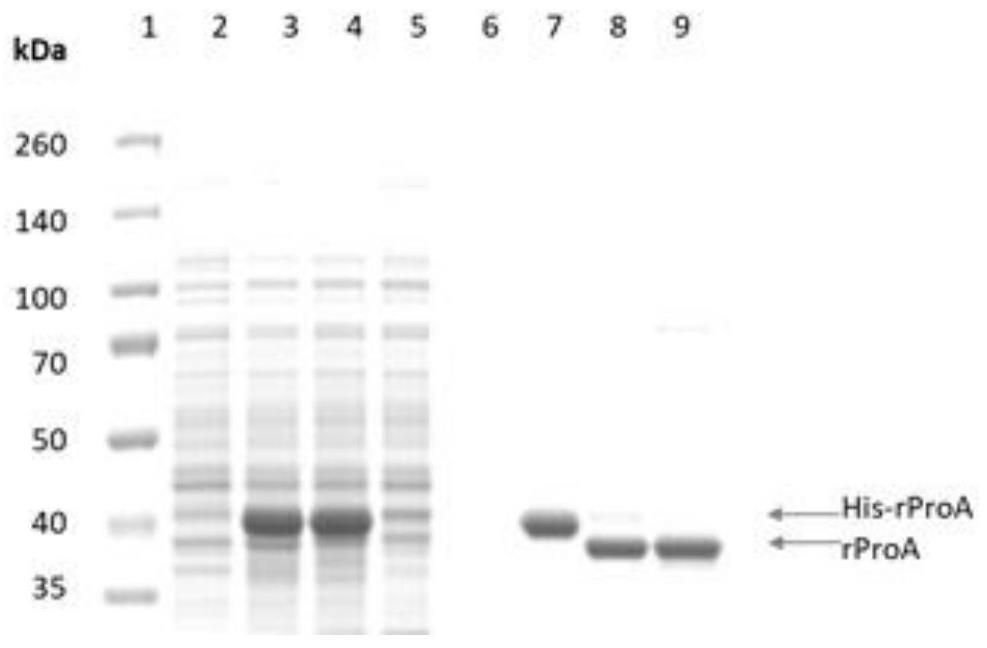
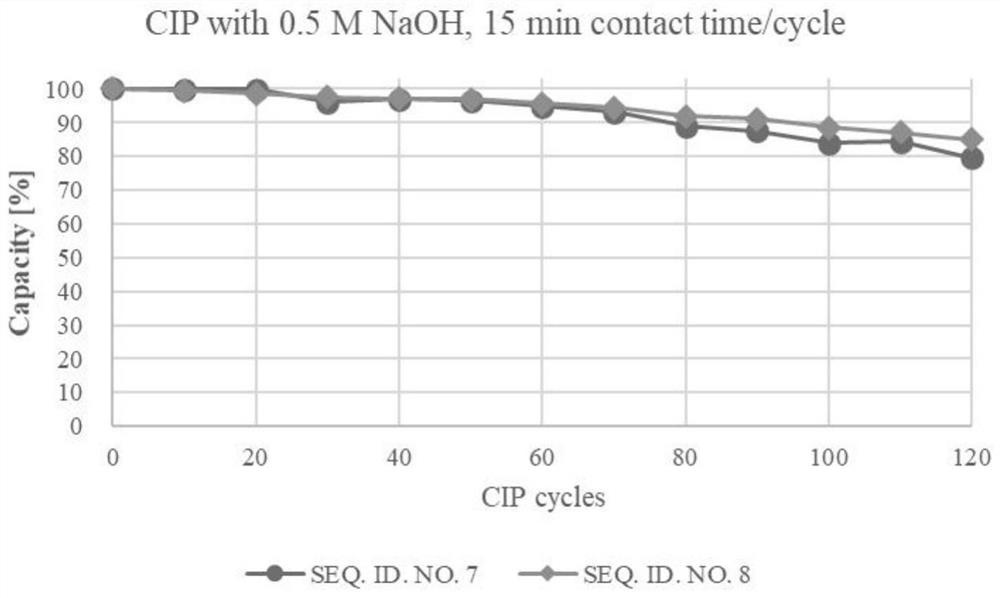
[0072] Production and purification of embodiment 2 recombinant protein A (recombinant protein A encoded by amino acid sequence SEQ ID NO.7)
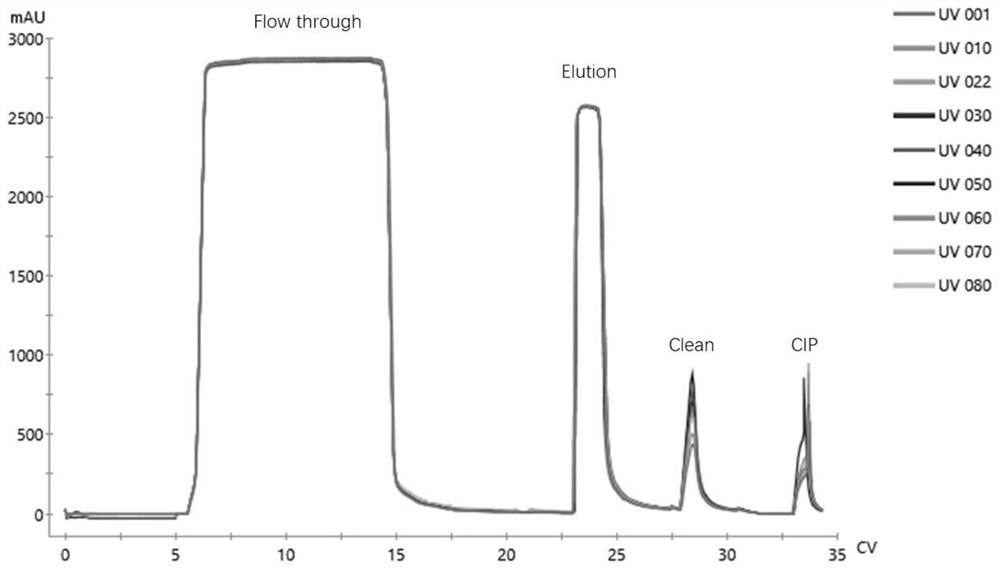
[0073] The production of recombinant protein A was carried out in a 10L fermenter, using LB medium, induced by 200mM IPTG, and confirmed whether the expression was successful by SDS-PAGE. After the end of expression, the cells were collected by centrifugation, and then the cells were resuspended in 1X PBS solution, followed by breaking the cells with a high-pressure homogenizer (18000 psi), and then centrifuging to remove cell debris. After clarification and filtration, the recombinant protein A was captured with a Ni affinity chromatography column. The chromatography medium was Polar MC60-Ni Excel (Sepax PN#: 270660800), and the column specification was 10cm (ID)*37cm (L). Impurities were rinsed with 2 times the column volume of 1X PBS solution after the sample, and the target protein was eluted with 0.1M phosphate buffer + 0.2M imidazo...
Embodiment 3
[0074] Example 3 Coupling of recombinant protein A and polymethacrylate microspheres
[0075] Activate hydrophilic polymethacrylate microspheres: 2L Monomix MC45-SEC (45μm, SepaxPN#: 280145950) ball into a 10L reactor, add 2L 1.5M NaOH and stir evenly, then react at 45°C and 150rpm for 1.5h, then add 2L 1,4-butanediol diglycidyl ether, continue After reacting for 1.5 h, the base balls were washed with ethanol and water respectively to obtain activated base balls.
[0076] Coupling of recombinant protein A produced in Example 2 to activated radical spheres: put 2L of activated radical spheres into a 10L reactor, then add 1L of 0.5M sodium carbonate solution and stir to disperse the activated radical spheres evenly, then add 1L of recombinant protein A solution (1X PBS solution containing 40 g of recombinant protein A in a reduced state), and finally 426 g of Na 2 SO 4And stir evenly, react at 30°C and 150rpm for 16h, add 10g of thioglycerol to continue the reaction for 3h a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com