Recombinant strain containing enol kinase gene and isopentenyl phosphokinase gene and application of recombinant strain in terpenoid production
A technology for isopentenyl phosphokinase and terpenoids, which is applied in the application field of terpenoid production, can solve problems such as enhancing the metabolic flux of precursors, and achieves the effects of facilitating rapid accumulation, efficient utilization, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
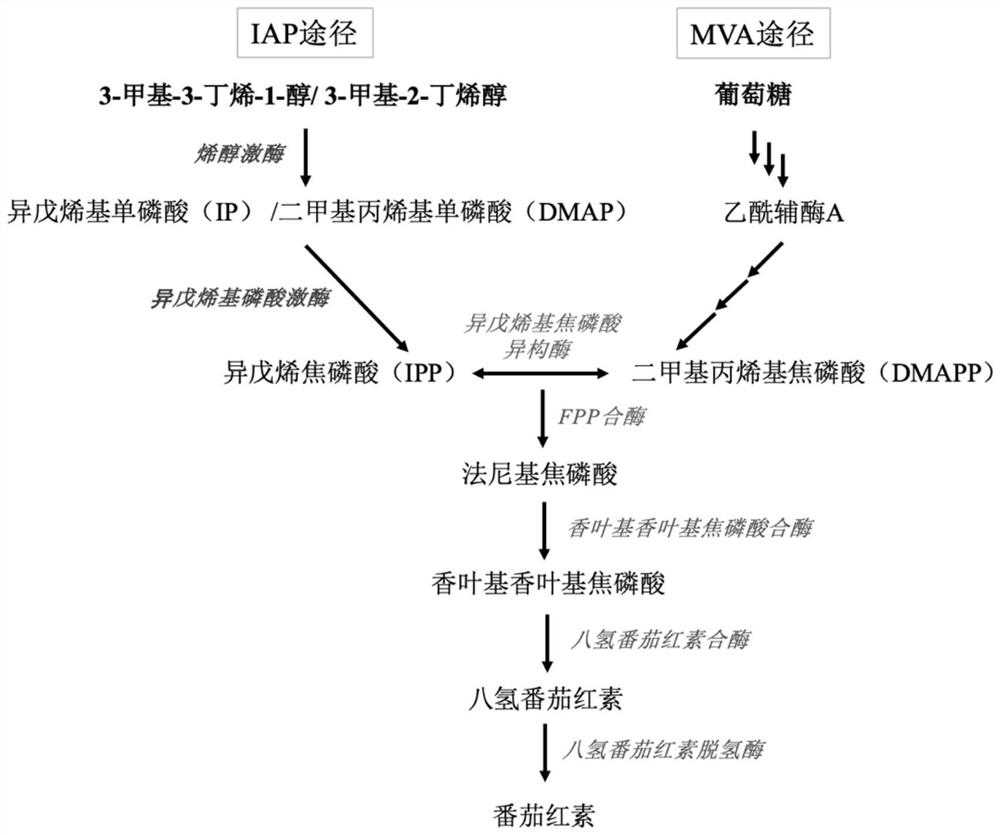
[0057] Example 1: Construction of yeast chassis for heterologous synthesis of tetraterpenoids (lycopene)
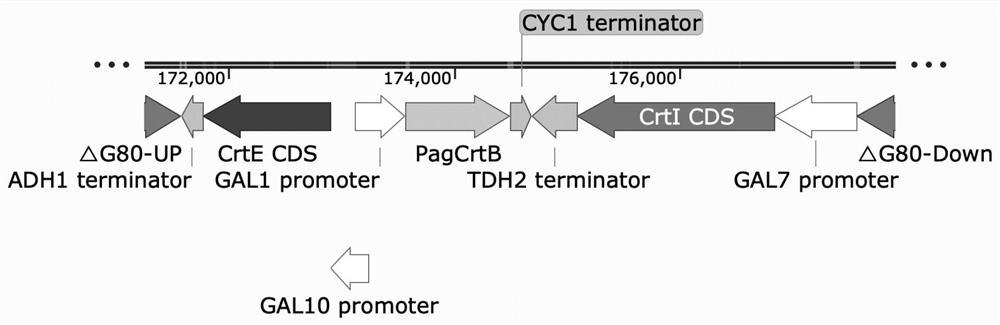
[0058] with high-fidelity enzymes ( Max Super-Fidelity DNA Polymerase) PCR to obtain the promoter (P GAL1 ,P GAL10 ,P GAL7 ), terminator (T ADH1 , T CYC1 , T TDH2 ) and the heterologous genes CrtE (geranylgeranyl pyrophosphate synthase, GGPP synthase), CrtB (phytoene synthase) and CrtI (phytoene dehydrogenase). By fusion PCR, the above elements were assembled into P GAL10 -CrtE-T ADH1 ,P GAL1 -CrtB-T CYC1 ,P GAL7 -CrtI-T TDH2 And construct the exogenous gene expression box CrtE-CrtB-CrtI, the structure is as follows figure 2 shown. Gel cutting and recovery to obtain the purified target fragment.
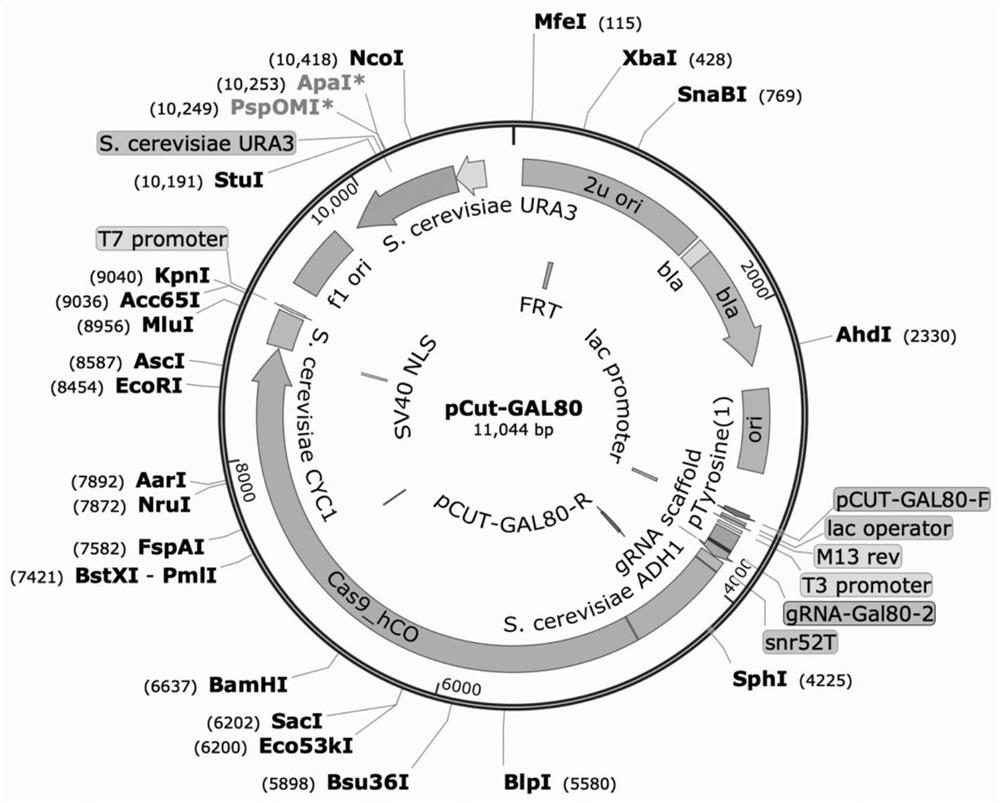
[0059] Using the CRISPR / Cas9 tool, the above exogenous gene expression cassette CrtE-CrtB-CrtI was integrated into the GAL80 position of the wild-type Saccharomyces cerevisiae CEN.PK2-1C genome to obtain the terpene-producing yeast chassis.
[0060] The knock-in...
Embodiment 2
[0073] Example 2: Assembly and expression of IAP pathway functional elements
[0074] Enol kinase (IAK) gene, phosphokinase (IPK) gene and bidirectional strong promoter P GAL10-GAL1 Assembled into an IAK-IPK expression cassette. The IAK-IPK expression cassette was recombined with the low-copy vector pRS415-LEU2 (CEN / ARS) using Gibson assembly technology to obtain the recombinant plasmid pRS415-IAK-IPK (structure as Figure 4 shown). These recombinant plasmids were transformed into the above-mentioned terpene-producing yeast chassis GLy80 for expression, and the transformation method was the same as in the above-mentioned Example 1.
[0075] The recombinant plasmids constructed in this example are: pRS415-ScCK-AtIPK and pRS415-SeCK-CsIPK.
[0076] Wherein, the specific genotype in the pRS415-ScCK-AtIPK plasmid combination is: the ScCK gene derived from Saccharomyces cerevisiae (Saccharomyces cerevisiae) (the nucleotide sequence is shown in SEQ ID NO: 1, and the amino acid se...
Embodiment 3
[0079] Example 3: Application of the Isopentenol Pathway (IAP) in Yeast Chassis
[0080] The yeast transformed in the above-mentioned Example 2 was spread on the plate for about 3 days to grow pink single colonies.
[0081] (1) Pick a single clone in a sterilized 24-well plate, load 2 mL of amino acid-deficient medium (SC-LEU+2% glucose) in each well of the 24-well plate, and culture overnight at 30° C., 800 rpm.
[0082] (2) Transfer the next day, take a certain amount of the above bacterial solution into a new 24-well plate. As above, each well of a 24-well plate was loaded with 2 mL of amino acid-deficient medium (SC-LEU+2% glucose). The initial OD600 after transfer was 0.2; cultured at 30°C, 800rpm for 24 hours.
[0083] (3) After 24 hours, it is measured that the above-mentioned bacterium grows to about OD600 of about 4-5, and the enol substrate Isoprenol and (or) Prenol are added to the medium with a final concentration of 25 mM, and then 10% dodecane ( V / V), that is,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap