Squaramide derivative covalent triazine skeleton polymer and application thereof in catalyzing coupling of carbon dioxide and epoxide to prepare cyclic carbonate
A technology of covalent triazine skeleton and cyclic carbonate, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, organic chemistry, etc., can solve the problem of difficult separation of homogeneous catalysts and products, High energy consumption, volatile organic solvents and other issues, to achieve the effects of excellent catalytic cycle performance, large industrial application potential, excellent catalytic activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation of embodiment 1 square amide monomer SAF-H
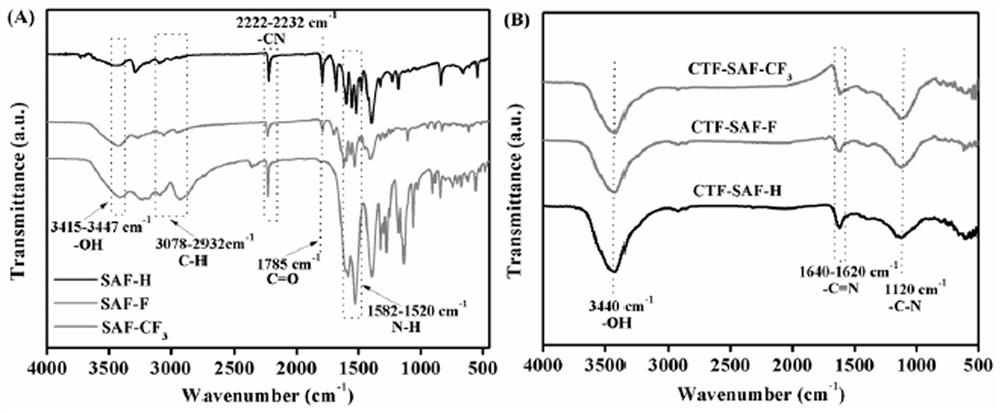
[0034]Weigh 5.2 mmol of diethyl squaraine into a round-bottomed flask, weigh 20 mL of a mixed solution of toluene and NMP, n=19:1, add 0.95 mmol of zinc trifluoromethanesulfonate, and weigh p-aminobenzonitrile (27.6 mmol). ) in a flask at 100°C for 48h of reaction. After the reaction, a solid-liquid mixture was obtained. The solid-liquid mixture was obtained by centrifugal filtration. The solid is the squaramide monomer, named SAF-H, and the yield is 93%.
[0035] The preparation of embodiment 2 square amide monomer SAF-F
[0036] Weigh 5.2 mmol of diethyl squaraine into a round-bottomed flask, weigh 20 mL of a mixed solution of toluene and NMP, n=19:1, add 0.95 mmol of zinc trifluoromethanesulfonate, and weigh 4-amino-3 fluoro- Benzonitrile (27.6 mmol) was stirred and reacted in a flask at 100 ° C for 48 h. After the reaction, a solid-liquid mixture was obtained. The mixture was centrifuged to filter the ob...
Embodiment 4
[0039] Example 4 Squaramide derivative covalent triazine skeleton CTF-SAF-H
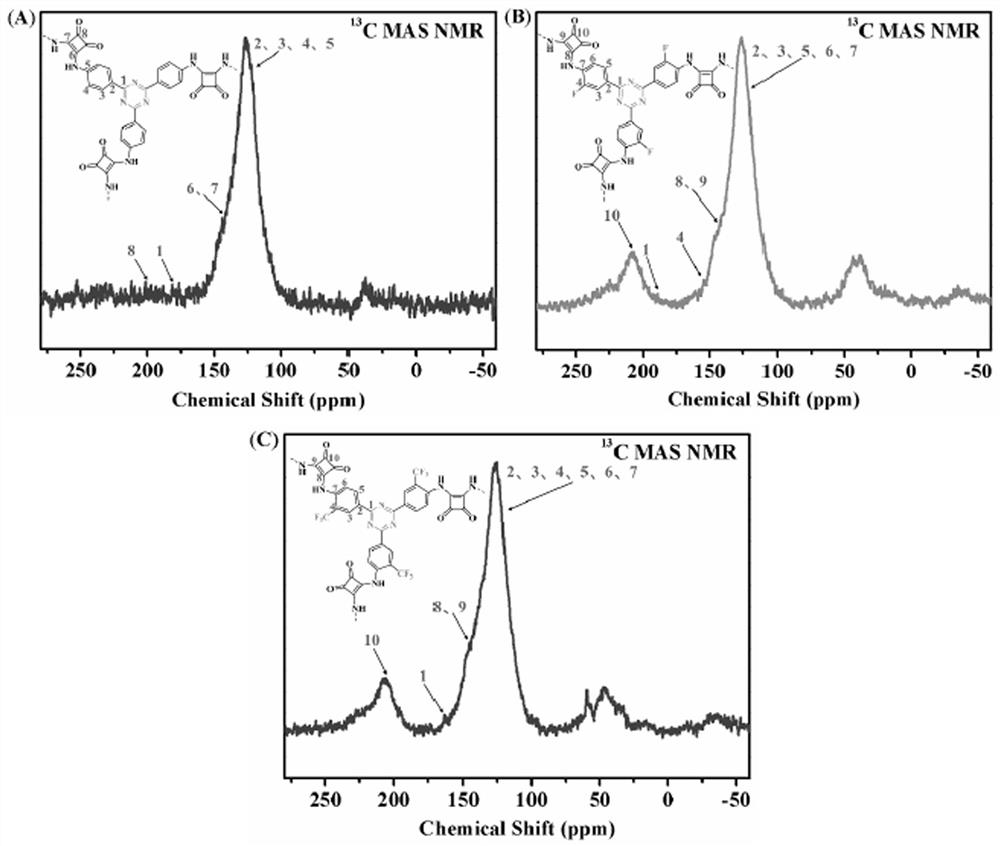
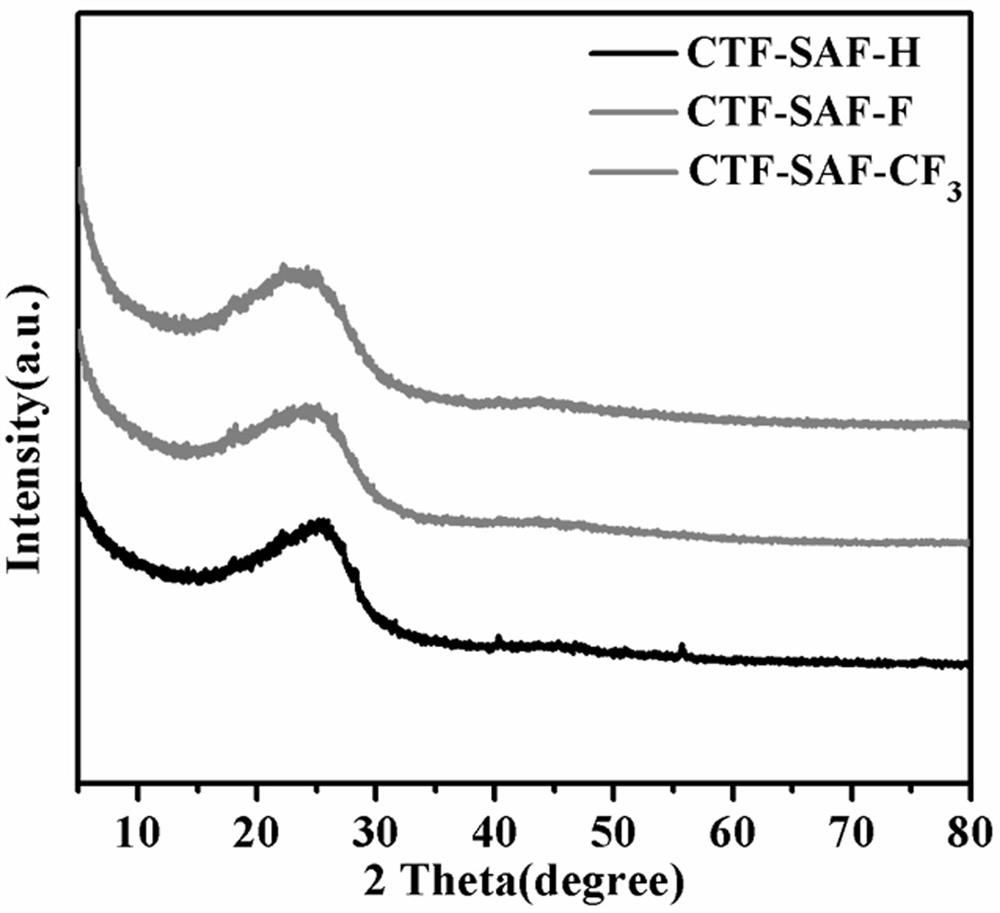
[0040] Weigh 1 mmol of SAF-H, the monomer of Squaramide, into a 10 ml ampoule tube, then weigh 10 mmol of zinc chloride and put it into the ampoule tube, vacuum the ampoule tube (10 min) and seal it, and place it in a muffle furnace at 400 °C for 40 h. , after the reaction, the polymer was transferred to a beaker, washed with distilled water (3×100mL), 0.1M HCl (3×30mL), methanol (3×30mL) in turn, and vacuum dried at 80°C for 24h to obtain black solid CTF-SAF-H , the yield is 48%.
Embodiment 5
[0041] Example 5 Squaramide derivative covalent triazine skeleton CTF-SAF-F
[0042] Weigh 1 mmol of the Squaramide monomer SAF-F into a 10 ml ampoule tube, then weigh 10 mmol of zinc chloride and put it into the ampoule tube, vacuum the ampoule tube (10 min) and seal it, and place it in a muffle furnace at 400 °C for 40 h roasting After the reaction, the polymer was transferred to a beaker, washed with distilled water (3×100mL), 0.1M HCl (3×30mL), methanol (3×30mL) successively, and vacuum dried at 80°C for 24h to obtain black solid CTF-SAF-F , the yield is 46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com