Green and efficient benzothiophene compound electrochemical synthesis method
A benzothiophene and synthesis method technology, applied in the field of electrochemical synthesis of benzothiophene compounds, can solve problems such as transition metal residues, poor selectivity, and difficult industrial scale-up, and achieve moderate reaction temperature, simple operation, and short reaction time short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
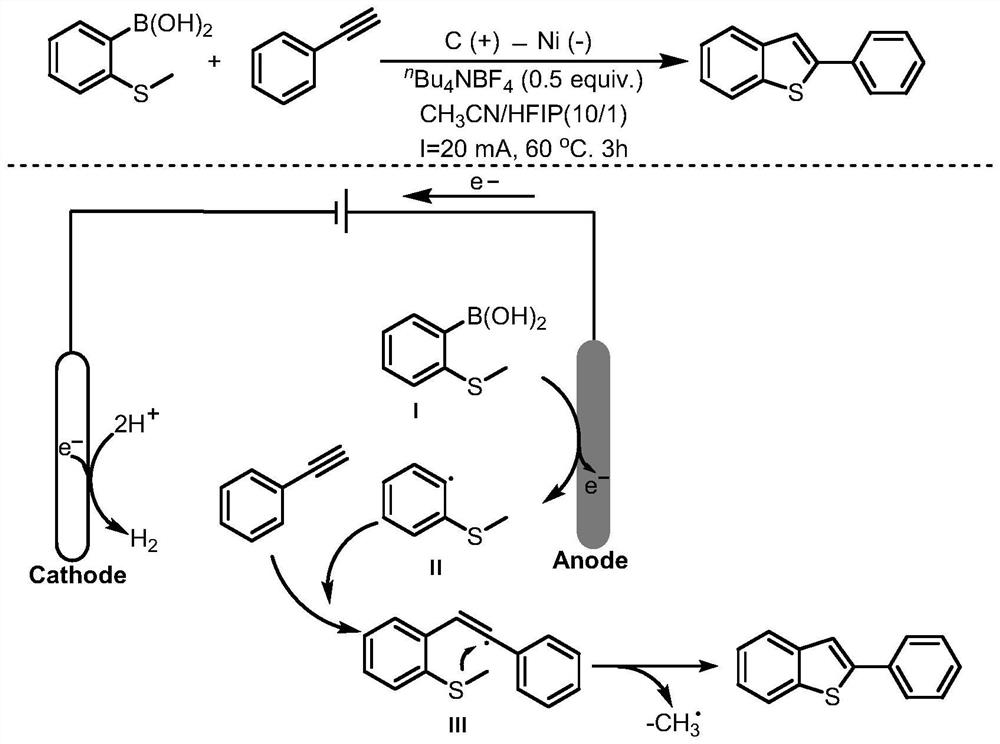
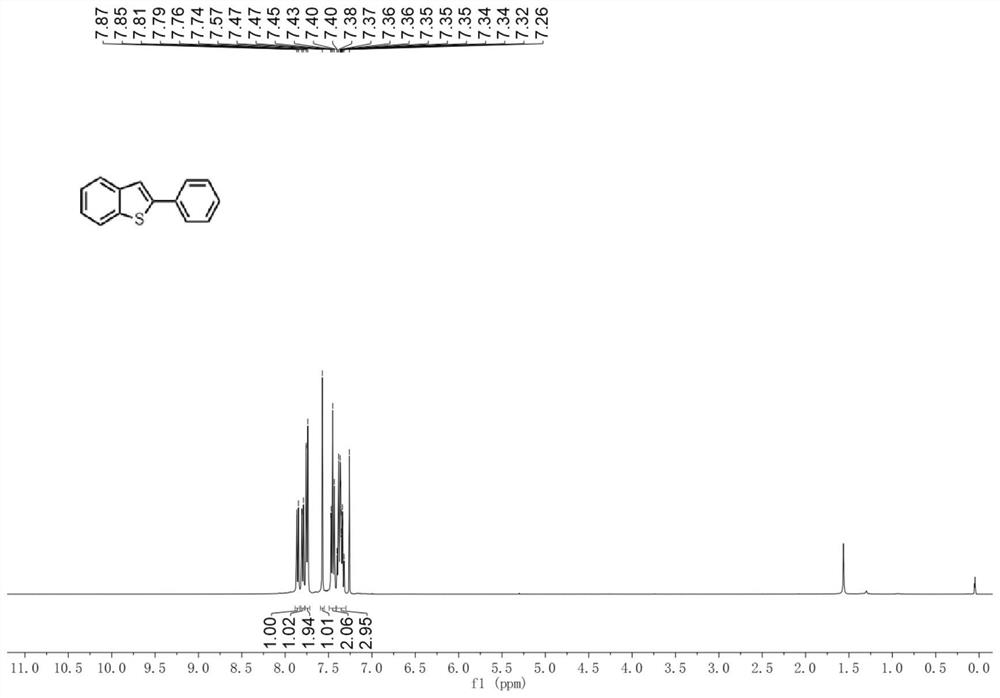
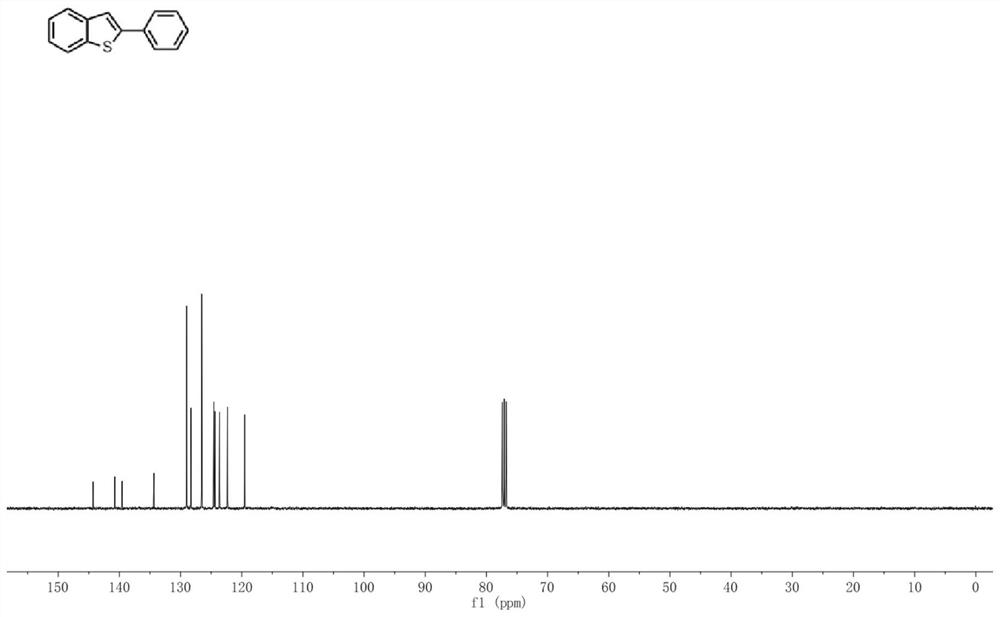
[0033] In a 50mL glass bottle equipped with an electrode, add o-methylthiophenylboronic acid (0.5mmol, 84.02mg), phenylacetylene (1.0mmol, 102.05mg), electrolyte tetrabutylammonium tetrafluoroborate (0.25mmol, 82.32mg) in turn ) and the reaction solvent acetonitrile (9mL) / acetic acid (1mL); the graphite rod electrode was used as the anode, the nickel electrode was used as the cathode to be connected to the DC power supply, and the reaction flask was placed in an oil bath at 60°C and stirred for 3 hours at a constant current of 20mA. ; cooled to room temperature, washed with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, concentrated, and separated and purified by silica gel column chromatography to obtain the target product 2-phenyl-benzothiophene with a yield of 96%. 1 H NMR (400MHz, CDCl 3 )δ7.86(d,J=7.7Hz,1H),7.80(d,J=7.1Hz,1H),7.75(d,J=7.0Hz,2H),7.57(s,1H),7.49–7.42( m,2H),7.41–7.30(m,3H). 13 C NMR(100MHz, CDCl3)δ144.30,140.75,139.5...
Embodiment 2
[0035] In a 50mL glass bottle equipped with an electrode, add o-methylthiophenylboronic acid (0.5mmol, 84.02mg), phenylacetylene (0.5mmol, 51.03mg), electrolyte tetrabutylammonium tetrafluoroborate (0.25mmol, 82.32mg) in turn ) and the reaction solvent acetonitrile (9mL) / acetic acid (1mL); the graphite rod electrode was used as the anode, the nickel electrode was used as the cathode to be connected to the DC power supply, and the reaction flask was placed in an oil bath at 60°C and stirred for 3 hours at a constant current of 20mA. ; cooled to room temperature, washed with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, concentrated, and separated and purified by silica gel column chromatography to obtain the target product 2-phenyl-benzothiophene with a yield of 81%.
Embodiment 3
[0037] In a 50mL glass bottle equipped with an electrode, add o-methylthiophenylboronic acid (0.5mmol, 84.02mg), phenylacetylene (1.5mmol, 153.08mg), electrolyte tetrabutylammonium tetrafluoroborate (0.25mmol, 82.32mg) in turn ) and the reaction solvent acetonitrile (9mL) / acetic acid (1mL); the graphite rod electrode was used as the anode, the nickel electrode was used as the cathode to be connected to the DC power supply, and the reaction flask was placed in an oil bath at 60°C and stirred for 3 hours at a constant current of 20mA. ; cooled to room temperature, washed with water, extracted with dichloromethane, dried over anhydrous sodium sulfate, filtered, concentrated, and separated and purified by silica gel column chromatography to obtain the target product 2-phenyl-benzothiophene with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com