Preparation and application of carrier-free double-drug self-assembled nanoparticles
A self-assembled nanoparticle, carrier-free technology, applied in the field of medicine, can solve the problems of catalysts and organic solvents and other toxic substances residues, complex preparation process, etc., to achieve the effect of improving drug bioavailability, reducing dosage, and improving inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Ursolic Acid and Sorafenib Carrier-Free Self-Assembled Nanoparticles
[0039] Accurately weigh 8 mg of ursolic acid powder, dissolve it in 2 mL of methanol, and configure it as a 4 mg / mL methanol solution of ursolic acid; accurately weigh 8 mg of sorafenib powder, dissolve it in 2 mL of methanol, and configure it as 4 mg / mL Sorafenib methanol solution; 4 mg / mL ursolic acid methanol solution and 4 mg / mL Sorafenib methanol solution were mixed uniformly according to the volume ratio of 4:1, as the organic phase; Add 0.0625 mL of organic phase dropwise to 1 mL of ultrapure water at a uniform speed, and ultrasonically disperse for 10–20 min at room temperature (25°C), ultrasonic frequency 40 kHz, and ultrasonic power 250 W, and then dry the methanol with a nitrogen blower. The aqueous solution of ursolic acid / sorafenib carrier-free self-assembled nanoparticles was obtained, and the nanoparticles were recorded as nanometer-1.
[0040]Accurately weigh...
Embodiment 2
[0044] Example 2 Observation of appearance and morphology of ursolic acid / sorafenib carrier-free self-assembled nanoparticles
[0045] Take 20 μL of the ursolic acid / sorafenib carrier-free self-assembled nanoparticle aqueous solution prepared in Example 1 and drop it on the surface of fresh mica sheet, and let it stand at room temperature for 30 min, and then wash it three times with ultrapure water to remove Surface impurities were finally blown dry with nitrogen, and images of the nanoparticle surface were collected under an atomic force microscope to confirm the morphology around the sample.
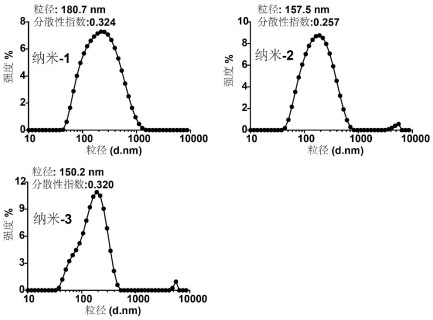
[0046] The result is as figure 2 As shown, the atomic force image results show that the morphology of nano-1, nano-2 and nano-3 is approximately spherical, and the dispersion is good.
Embodiment 3
[0047] Example 3 Stability investigation of ursolic acid / sorafenib self-assembled nanoparticles without carrier
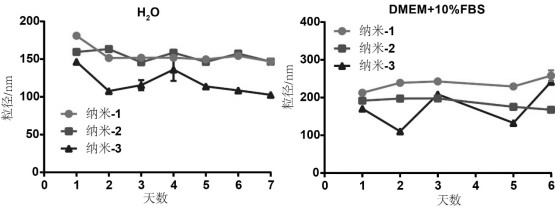
[0048] The ursolic acid / Sorafenib carrier-free self-assembled nanoparticles prepared in Example 1 were dispersed in ultrapure water or in DMEM medium containing 10% fetal bovine serum (FBS) by volume fraction, at 4°C Stored under low temperature, take out an equal volume of nanometer solution every day, measure its particle size with a dynamic light scattering particle size analyzer and record the data.
[0049] The result is as image 3 As shown, the stability results show that when ursolic acid / Sorafenib carrier-free self-assembled nanoparticles are dispersed in ultrapure water and DMEM+10%FBS, the particle size still maintains a relatively stable state, which can be Subsequent cell experiments will provide the experimental basis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com