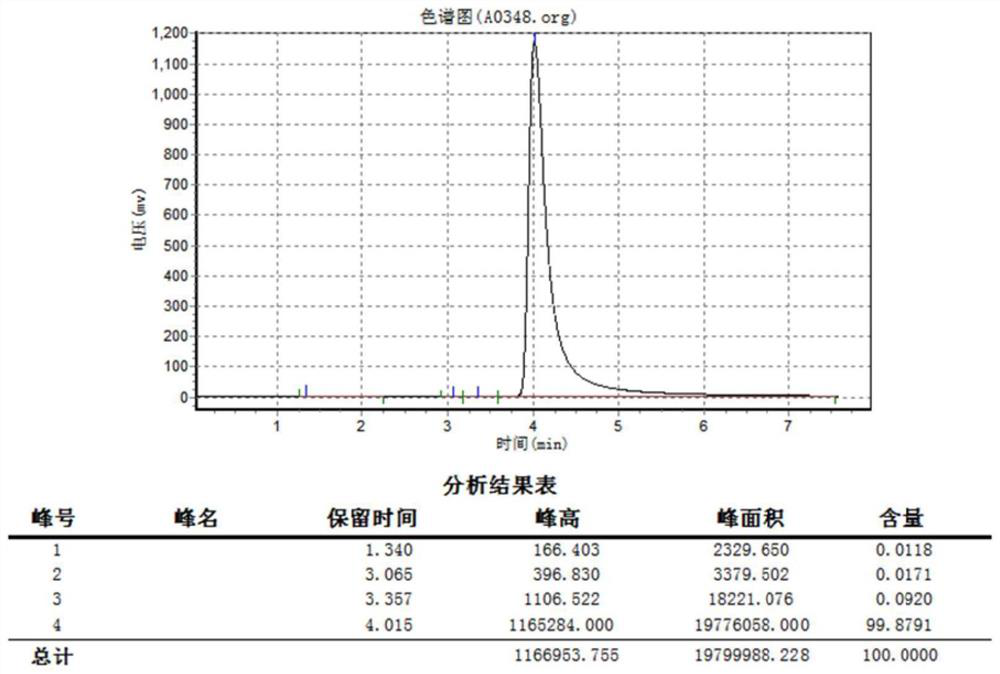
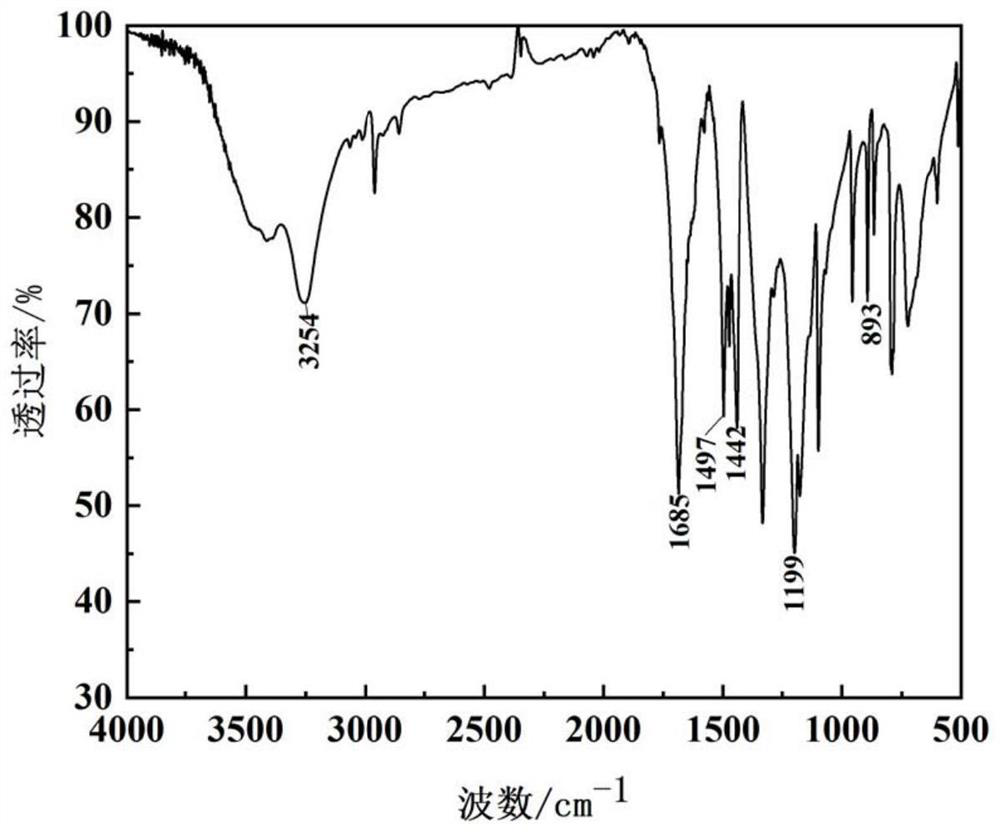
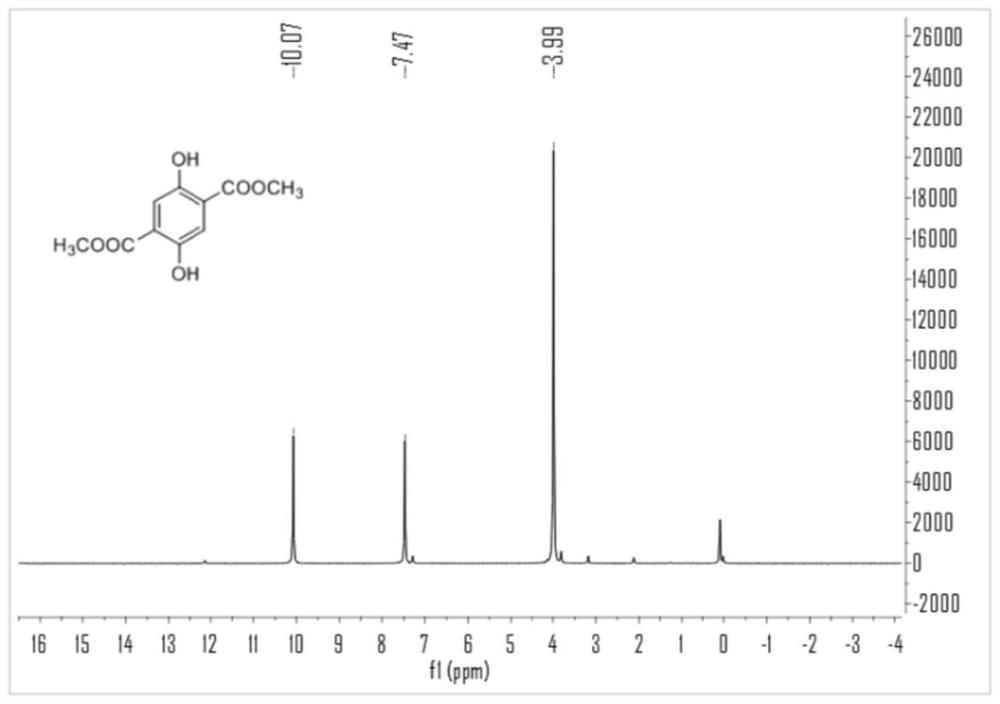
Method for synthesizing dihydroxy dimethyl terephthalate by using oxygen catalytic oxidation method
A technology of dimethyl dihydroxyterephthalate and dimethyl hydroxyterephthalate, which is applied in the field of organic chemical synthesis, can solve the problems of three-waste discharge, high cost, low yield and the like, and achieves reduction of three-waste discharge and production The effect of simplifying the process and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Co / N-C preparation
[0030] (1) Mix 2g of cobalt acetate tetrahydrate, 15g of glucose, 4g of urea, and 3g of water, and mix well to dissolve the cobalt acetate to obtain a red viscous liquid;
[0031] (2) Place the prepared red viscous liquid in a tube furnace and feed NH 3 , the temperature was raised to 800°C at a rate of 1°C / min, and maintained at 800°C for 2 hours, then the temperature was naturally lowered to 40°C, and the material was taken out to obtain 5.2g of Co / N-C catalytic material.
[0032] The BET specific surface area is 98㎡ / g and the pore volume is 0.32ml / g as determined by liquid nitrogen adsorption method. The content of surface elements Co: 10.1%, N: 6.3% was analyzed by scanning electron microscope surface energy spectroscopy (SEM-EDS).
Embodiment 2
[0033] Embodiment 2: Cu / N-C preparation
[0034] (1) Fully mix 3g of copper chloride dihydrate, 15g of glucose, 5g of urea, and 4g of water to dissolve the copper chloride to obtain a green viscous liquid.
[0035] (2) The prepared green viscous liquid is placed in a tube furnace and fed with NH 3 , the temperature was raised to 700°C at a rate of 3°C / min, and maintained at 700°C for 4 hours, then the temperature was naturally lowered to 40°C, and the material was taken out to obtain 5.8g of Cu / N-C catalytic material.
[0036] The BET specific surface area is 79㎡ / g and the pore volume is 0.29ml / g as determined by liquid nitrogen adsorption method. Surface element content Cu: 18.6%, N: 4.4% were analyzed by scanning electron microscope surface energy spectroscopy (SEM-EDS).
Embodiment 3
[0037] Embodiment 3: Ni / N-C preparation
[0038] (1) Fully mix 4g of nickel nitrate hexahydrate, 15g of glucose, 6g of urea, and 4g of water to dissolve the nickel nitrate to obtain a green viscous liquid.
[0039] (2) The prepared green viscous liquid is placed in a tube furnace and fed with NH 3 , the temperature was raised to 850°C at a rate of 5°C / min, and maintained at 850°C for 3 hours, then the temperature was naturally lowered to 35°C, and the material was taken out to obtain 5.4g of Ni / N-C catalytic material.
[0040] The BET specific surface area is 116㎡ / g and the pore volume is 0.35ml / g as determined by liquid nitrogen adsorption method. Surface element content Ni: 14.7%, N: 4.7% were analyzed by scanning electron microscope surface energy spectroscopy (SEM-EDS).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com