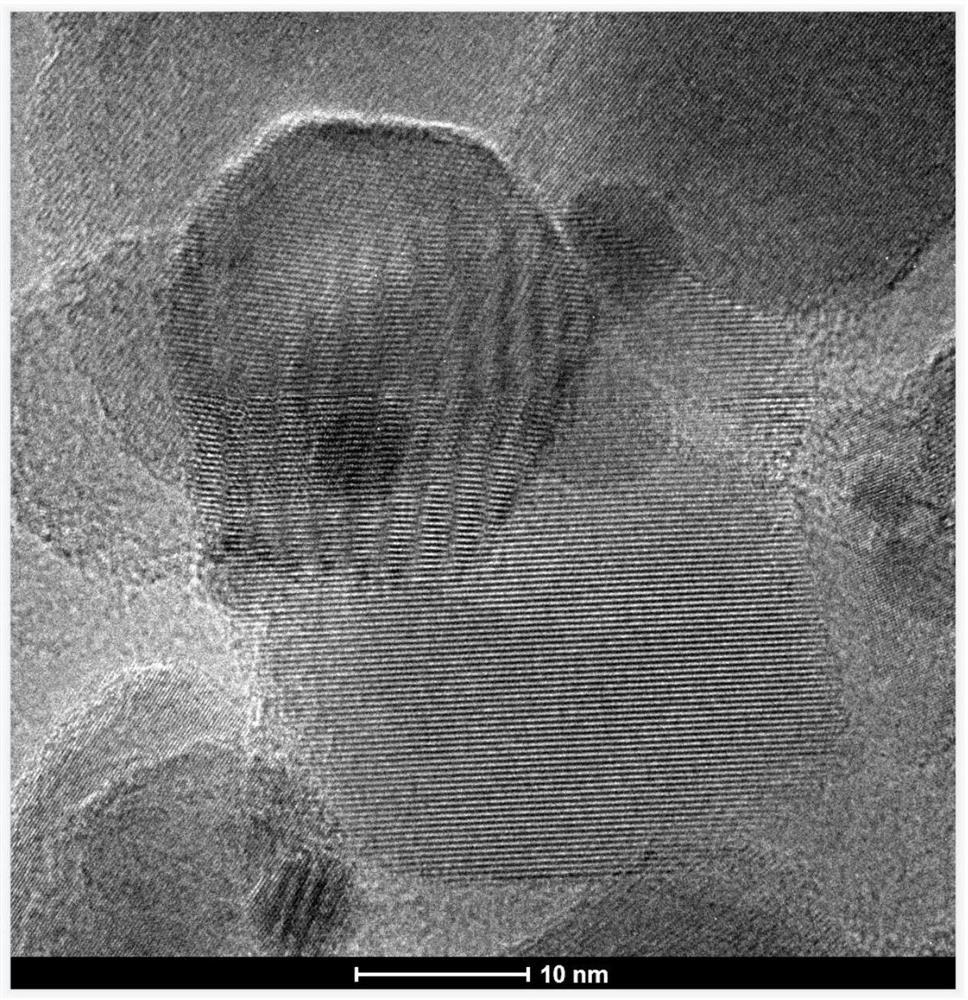
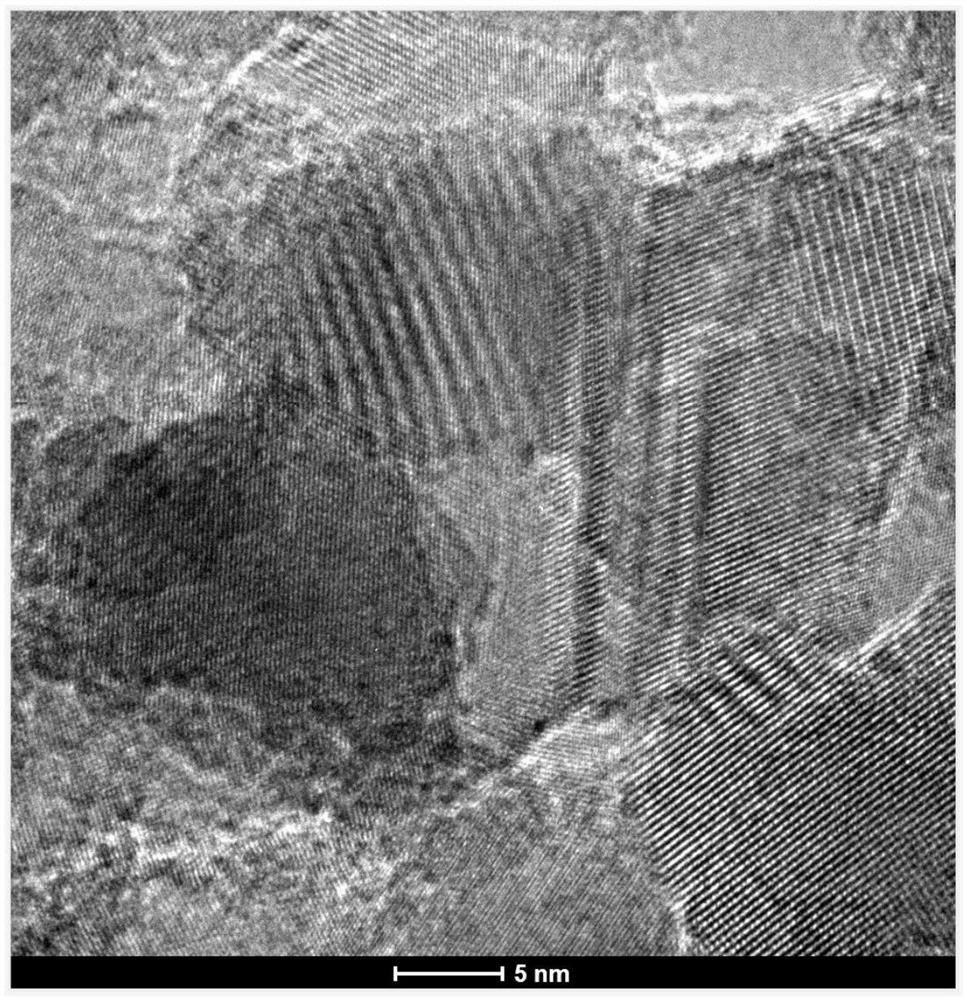
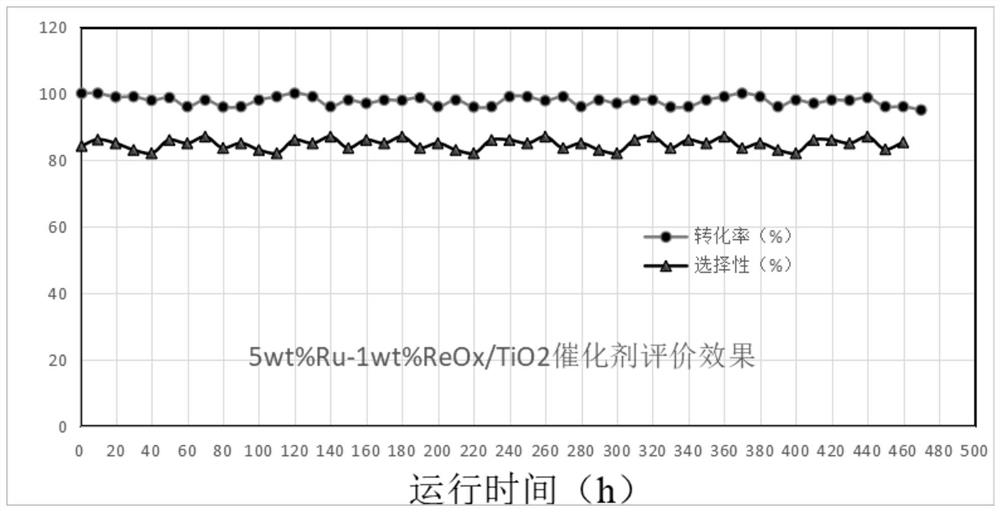
Method for continuously synthesizing homopiperazine
A technology of homopiperazine and mixed gas, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Problems such as recycling and regeneration, synthetic routes cannot be reproduced repeatedly, etc., to achieve the effects of stable activity, good catalytic effect and long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] The preparation method of the catalyst may be a precipitation method, an impregnation method, a blending method, a sol-gel method; preferably a precipitation method and an impregnation method.
[0084]Preferably, the preparation method of the catalyst is carried out as follows: the catalyst active component precursor metal hydrochloride or nitrate (selected from ruthenium (Ru), platinum (Pt), rhodium (Rh)) or gold (Au) Sulfate or nitrate is dissolved in water or alcohol to form a solution, and the active component is deposited and precipitated by forward dripping with lye at 20-80°C. When the pH of the system reaches 2-5, the carrier powder is added, and then the second Second active component metal solution (such as ammonium perrhenate, manganese acetate, etc.), the concentration of the second active component solution is controlled at 0.05-0.5mol / L, continue to titrate the lye until the pH of the system reaches 12-13, fully stir beating and aging 3-12 hours, after agi...
preparation Embodiment 1
[0089] Preparation Example 1: 5wt%Ru-1wt%ReOx / TiO 2 Preparation of:
[0090] Weigh 100g of TiO2 (P25, Evonik Degussa, Germany) and vacuum degass at 150°C for 12h before use. 13.5g of ruthenium trichloride hydrate (Ru content 37.5%, Sinopharm Chemical Reagent Co., Ltd.) was dissolved in 50ml of water to form a catalyst precursor solution, the catalyst precursor solution was heated to 40°C, and 10% ammonia water was added dropwise forward to make When the pH of the system reaches 2.5, put in the catalyst carrier powder, stir and impregnate for 3 hours; dissolve 1.5g of ammonium perrhenate (purchased from Beijing Bailingwei Technology Co., Ltd.) in 20ml of water, add the above slurry, and continue to titrate 10% ammonia water until the pH of the system reaches 12. Fully stir and beating and aging for 12 hours. After aging, filter and wash the slurry until the filtrate becomes neutral. After adding the forming auxiliary scallop powder, the slurry is extruded into 3mm round bars o...
preparation Embodiment 2
[0091] Preparation Example 2: 5wt%Ru-10wt%ReOx / TiO 2 preparation of
[0092] The preparation method is the same as that of Catalyst Preparation Example 1, except that the amount of ammonium perrhenate is increased to 15 g. The catalyst prepared according to this method is labeled as 5wt%Ru-10wt%ReOx / TiO 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com