Postbiotics as well as preparation method and application thereof
A technology of epigenetics and strains, applied in biochemical equipment and methods, methods based on microorganisms, bacteria used in food preparation, etc., can solve the problems of unclear functions of postbiotics, poor stability and dispersion, unstable product performance, etc. problem, to achieve the effect of shortening the production cycle, good thermal stability, and shortening the cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
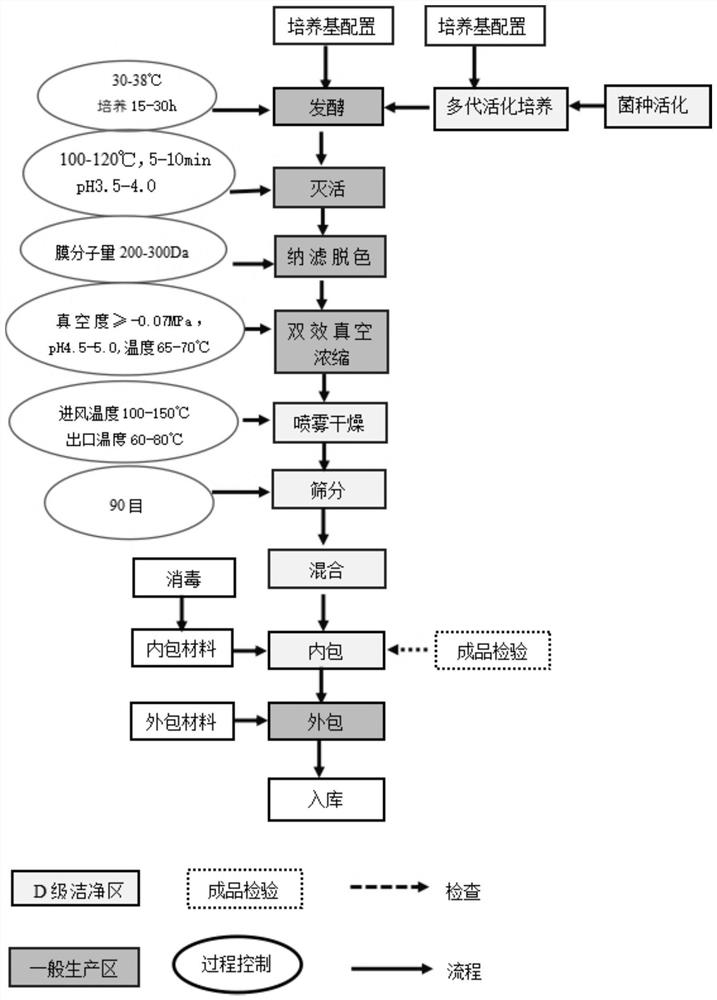
[0064] refer to Figure 1-10 , a postbiotic of the present embodiment, said postbiotic includes inactivated Lactobacillus plantarum LP220 and its fermentation metabolites, and the preservation number of said Lactobacillus plantarum LP220 is CCTCC NO: M2018465.
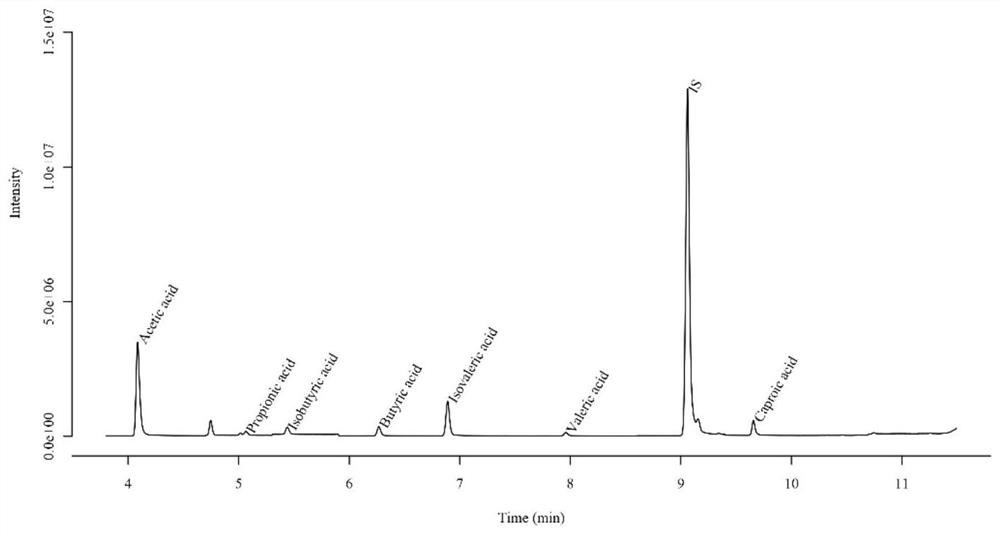
[0065] The composition of the postbiotics includes: Lactobacillus plantarum LP220 is 1.2*10 11 CFU / g (number of bacteria ≥ 1*10 11 CFU / g), the content of lipoteichoic acid is 36.2ng / kg, the total content of short-chain fatty acids is 99.97μg / g, the content of polypeptide is 6.0%, and the small molecule peptide with molecular weight <1000DA accounts for 89.1% of the total peptide content , the organic acid content is 127mg / g.
[0066] The preparation method of a kind of postbiotic of the present embodiment comprises the following steps:
[0067] S1, strain activation
[0068] Sterilization of all used utensils: need to be placed in a pressure steam sterilizer, sterilized at 121°C for 25-30 minutes before use.
[00...
Embodiment 2
[0167] A postbiotic of this embodiment, said postbiotic includes inactivated Lactobacillus plantarum LP220 and its fermentation metabolites, and the preservation number of said Lactobacillus plantarum LP220 is CCTCC NO: M2018465. The composition of the postbiotics includes: the number of Lactobacillus plantarum LP220 is 2*10 11 CFU / g, the content of lipoteichoic acid is 35.7ng / kg, the content of short-chain fatty acid is 96.12μg / g, the content of polypeptide is 6.25%, and the small molecule peptide with molecular weight <1000DA accounts for 88.4% of the total peptide content, organic acid The content is 116mg / g.
[0168] The preparation method of a kind of postbiotic of this embodiment, and the preparation method of embodiment 1, there is following difference:
[0169] S2, fermentation and inactivation
[0170]Transfer about 15,000ml of the third-generation activated culture strain to a cleaned and sterilized fermenter according to the inoculum size of 6.0% (V / V). The temper...
Embodiment 3
[0174] A postbiotic of this embodiment, said postbiotic includes inactivated Lactobacillus plantarum LP220 and its fermentation metabolites, and the preservation number of said Lactobacillus plantarum LP220 is CCTCC NO: M2018465. The composition of the postbiotics includes: the number of Lactobacillus plantarum LP220 is 1.5*10 11 CFU / g, the content of lipoteichoic acid is 35.1ng / kg, the content of short-chain fatty acid is 93.12μg / g, the content of polypeptide is 6.62%, and the small molecule peptide with molecular weight <1000DA accounts for 85.8% of the total peptide content, organic acid The content is 110mg / g.
[0175] The preparation method of a kind of postbiotic of this embodiment, and the preparation method of embodiment 1, there is following difference:
[0176] S2, fermentation and inactivation
[0177] Transfer about 20,000ml of the third-generation activated culture strain to a cleaned and sterilized fermenter according to the inoculum size of 5% (V / V). In the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com