Porous gel electrolyte and preparation method and application thereof
A gel electrolyte and electrolyte technology, applied in the field of polymers, can solve problems such as carbon emissions, and achieve the effects of increased energy density, high electrolyte absorption, and adjustable porosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The invention provides a method for preparing a porous gel electrolyte, comprising the following steps:
[0051] S1. Add polylactic acid (PLA) to dichloromethane (DCM) and magnetically stir to obtain product A. Add polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) to N,N-dimethylformamide (DMF ) was magnetically stirred to obtain product B, and after fully dissolving, product B (PVDF-HFP solution) was added to product A (dichloromethane solution of PLA), and stirred again to obtain a uniform mixed solution (electrolyte slurry);
[0052] The mass of polylactic acid is 80% to 90% of the total mass of polyvinylidene fluoride-hexafluoropropylene and polylactic acid, and the mass of polyvinylidene fluoride-hexafluoropropylene is 80% to 90% of the total mass of polyvinylidene fluoride-hexafluoropropylene and polylactic acid. 10%~20%, the volume of dichloromethane is 30%~70% of the total volume of dichloromethane and N,N-dimethylformamide, the volume of N,N-dimethylformam...
Embodiment 1
[0063] (1) Weigh 0.58128 mg of polylactic acid (PLA), add it to 2.1 mL of dichloromethane (DCM), and stir magnetically at 500 rpm for 4 hours; weigh 0.14532 mg of polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) mg, added to 0.9mL N,N-dimethylformamide (DMF), magnetically stirred at 500rpm for 4 hours;
[0064] (2) The solution of PVDF-HFP obtained in step 1 was added to the methylene chloride solution of PLA, and magnetically stirred at 600 rpm for 4 hours, allowing the two solutions to be fully mixed;
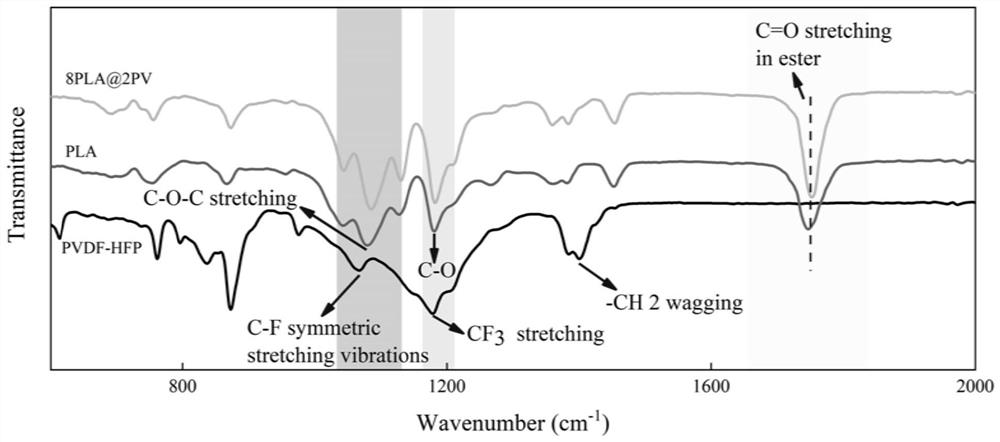
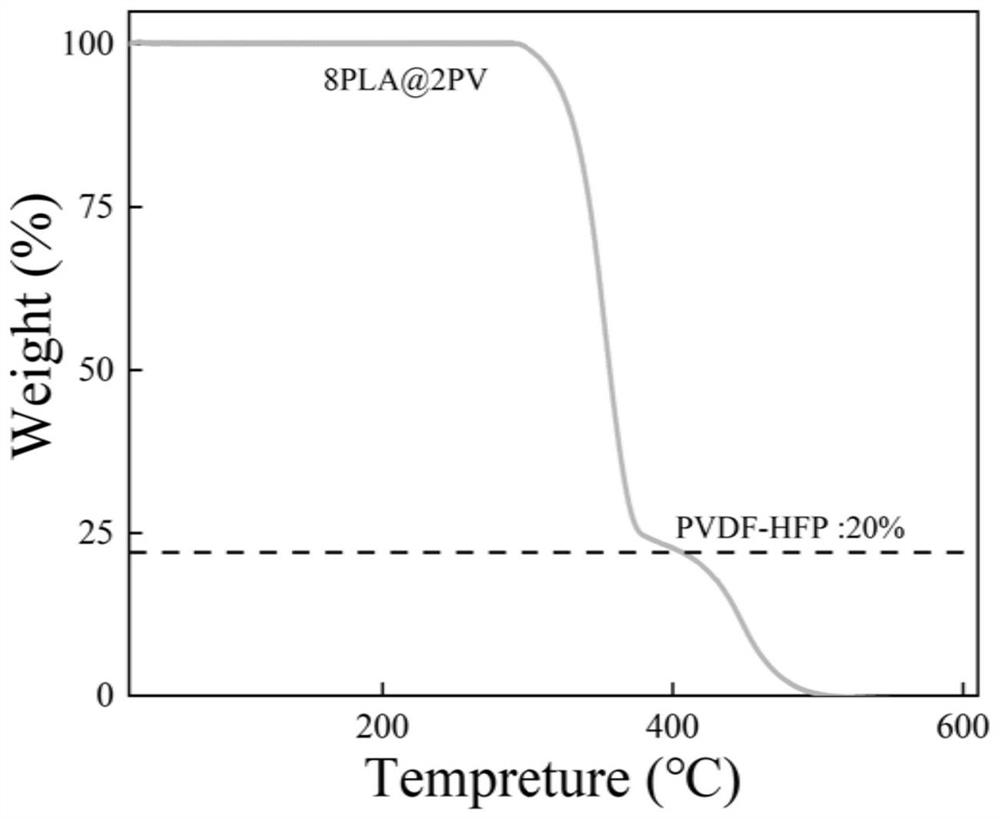
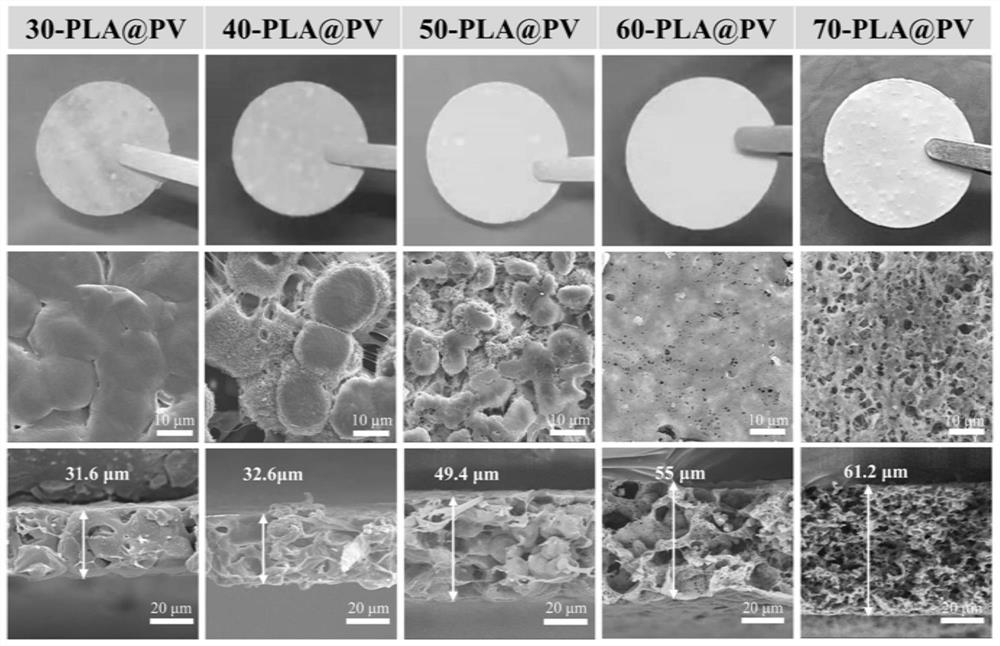
[0065] (3) Decoat the mixed solution obtained in step 2 on the polytetrafluoroethylene film with a 200 μm scraper, place it in a fume hood at room temperature for 2 hours to volatilize the dichloromethane to form a film; keep it in a vacuum oven at 60°C for 8 hours, volatilize DMF pore formation, named 30DMF-PLA@PV. The prepared electrolyte was soaked in electrolyte solution for 4 hours, and then packaged and assembled button-type and quasi-solid supercapacitors.
Embodiment 2
[0067] (1) Weigh 0.56304 mg of polylactic acid (PLA), add it to 1.8 mL of dichloromethane (DCM), and stir magnetically at 500 rpm for 4 hours; weigh 0.14076 mg of polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) mg, added to 1.2mL N,N-dimethylformamide (DMF), magnetically stirred at 600rpm for 4 hours;
[0068] (2) The solution of PVDF-HFP obtained in step 1 was added to the methylene chloride solution of PLA, and magnetically stirred at 600 rpm for 4 hours, allowing the two solutions to be fully mixed;
[0069] (3) Decoat the mixed solution obtained in step 2 on the polytetrafluoroethylene film with a 200 μm scraper, place it in a fume hood at room temperature for 2 hours to volatilize the dichloromethane to form a film; keep it in a vacuum oven at 60°C for 8 hours, volatilize DMF pore formation, named 40DMF-PLA@PV. The prepared electrolyte was soaked in electrolyte solution for 4 hours, and then packaged and assembled button-type and quasi-solid supercapacitors.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com