Synthesis method of tert-butyl (meth) acrylate
A technology for the synthesis of tert-butyl acrylate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve the problem of high 78%, selectivity not mentioned, catalyst and by-product water treatment difficulties , did not mention the problem of polymerization inhibitor system, etc., to achieve the effect of improving conversion rate and selectivity, inhibiting side reactions of polymerization, and reducing steam energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
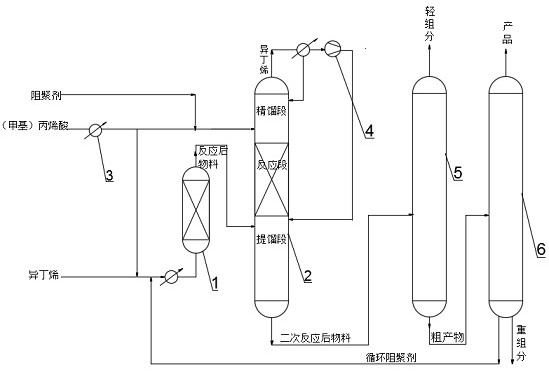
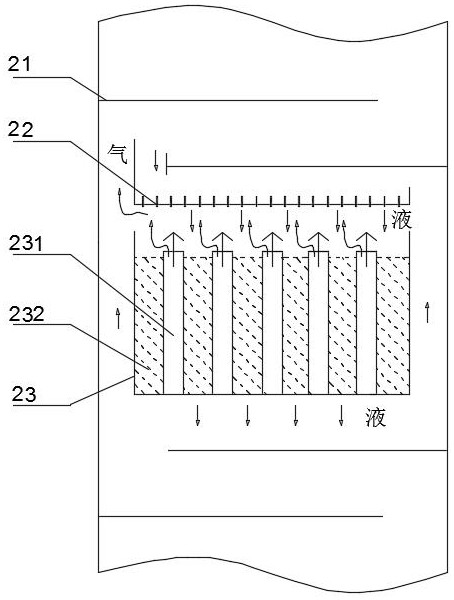
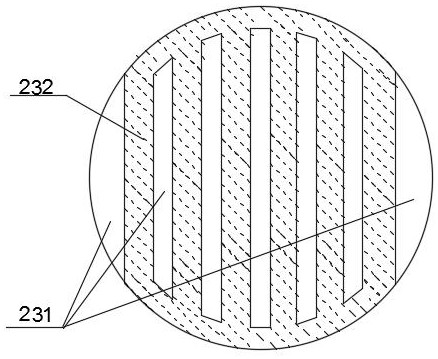
[0040] Use resin catalyst QRE-02 (particle size is 0.3-1.1mm, cross-linking degree is about 10-20%, exchange capacity is greater than 4.0mmolH + / g (干) above. ) is the catalyst 232 of the esterification reaction, selects HQ and MEHQ as polymerization inhibitor, wherein the molar ratio of HQ and MEHQ is 2:1; Adopt fixed bed reactor 1 as the first reactor, adopt as Figure 2-3 The shown reactive distillation tower 2 is used as the second reactor, and the vacuum distillation tower is used as the light removal tower 5 and the weight removal tower 6; the catalyst module 23 of the reactive distillation tower 2 is equipped with a catalyst 232, and the amount of the catalyst 232 is 100ml. The structural parameters of the reactive distillation tower 2 are that the rectification section is 2 to 5 theoretical trays, the reaction section is 6-10 theoretical trays, the stripping section is 11 to 20 theoretical trays, and the catalyst The number of modules 23 is 4 to 10. A liquid distrib...
Embodiment 2
[0047] Use modified resin Amberlyst A35 (particle size is 0.3-1.1mm, cross-linking degree is about 10-20%, exchange capacity is greater than 4.0mmolH + / g (干) above. ) is the catalyst 232 for the esterification reaction, and HQ and 4-hydroxyl-2,2,6,6-tetramethylpiperidine-1-oxyl radical are selected as polymerization inhibitors, wherein HQ and 4-hydroxyl-2,2 , the molar ratio of 6,6-tetramethylpiperidine-1-oxyl radical is 1:1; adopt fixed bed reactor 1 as the first reactor, adopt as Figure 2-3 The shown reactive distillation tower 2 is used as the second reactor, and the vacuum distillation tower is used as the light removal tower 5 and the weight removal tower 6; the catalyst module 23 of the reactive distillation tower 2 is equipped with a catalyst 232, and the amount of the catalyst 232 is 100ml. The structural parameters of the reactive distillation tower 2 are that the rectification section is 2 to 5 theoretical trays, the reaction section is 6-10 theoretical trays, th...
Embodiment 3
[0055] In the first reactor: pressure 0.1~0.5MPa, temperature 10~40℃, liquid space velocity 0.5~5.0h -1 , the acid-ene ratio is 1.0-5.0 mol / mol;
[0056] Inside the reactive distillation tower 2: pressure -0.02~0.1MPa, reaction section temperature 20~45°C, acid-ene ratio 2.0~6.0: 1mol / mol;
[0057] Light removal tower 5: pressure -0.01~-0.09MPa, tower top temperature 45~78℃, reflux ratio 5~20;
[0058] Weight removal tower 6: pressure -0.07~-0.10MPa, tower top temperature 48~73℃, reflux ratio 5~15;
[0059] The parameters involved in the first reactor, reactive distillation tower 2, light removal tower 5 and weight removal tower 6 are arbitrarily selected and combined within the above range; other operations are the same as in embodiment 1 or embodiment 2. The isobutene reacts completely.
[0060] The product extracted from the top of the weight-removing tower 6 is detected by a chromatograph:
[0061] The content of tert-butyl acrylate is 99.3-99.7%, and the selectivity o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com