Cryptolepis sinensis zinc (II) complex with in-vivo and in-vitro high activity as well as synthesis method and application thereof
The invention relates to a technology for zinc and a synthesis method of vine leaves, which can be applied in the field of medicine and can solve the problems of restricting the curative effect and long-term use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Ligand BQTC:
[0039] Weigh 1.00mol of 1,4,7-tri-tert-butoxycarbonyl-1,4,7,10-tetraazacyclododecane, 1.20mol of 7-hydroxyheptanoic acid, 2.00mol of 1-ethyl-3( 3-Dimethylpropylamine) carbodiimide (EDCI) and 5.0 mL of pyridine were reacted at 70.0 ° C for 16.0 hours to obtain yellow compound 1 with a yield of 28.8%; Compound 1, 1.20 mol of compound BQ and 5.00 mol of NaH were dissolved in 5.0 mL of dimethylacetamide solution (DMA) and reacted at 100.0 °C for 3.0 hours to obtain yellow compound 2 with a yield of 32.5%; Add 5.00mol HCl / dioxane solution (HCl / dioxane) and 5.0mL CH to 1.00mol compound 2 2 Cl 2 (DCM), reacted at 25.0° C. for 16.0 hours, and passed through the column to obtain the yellow ligand BQTC with a yield of 53.5%.
[0040] Identification of the resulting product:
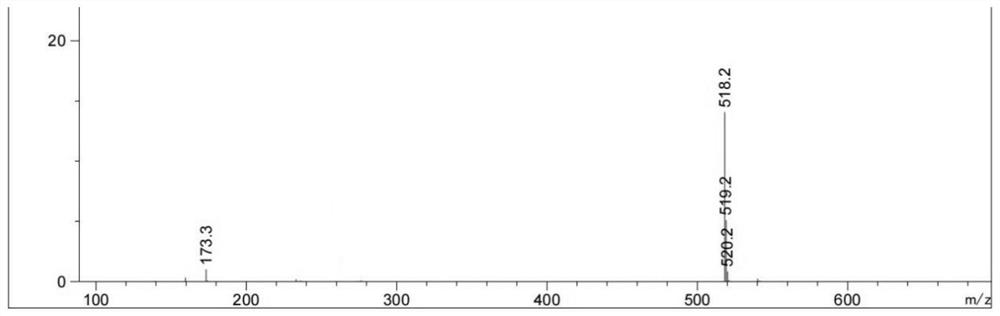
[0041] (1) Electrospray mass spectrometry of compound 1, its spectrum is as follows figure 1 shown.
[0042] ESI-MS m / z: 601.5[M+H] + , where M is the molecular weight of c...
Embodiment 2
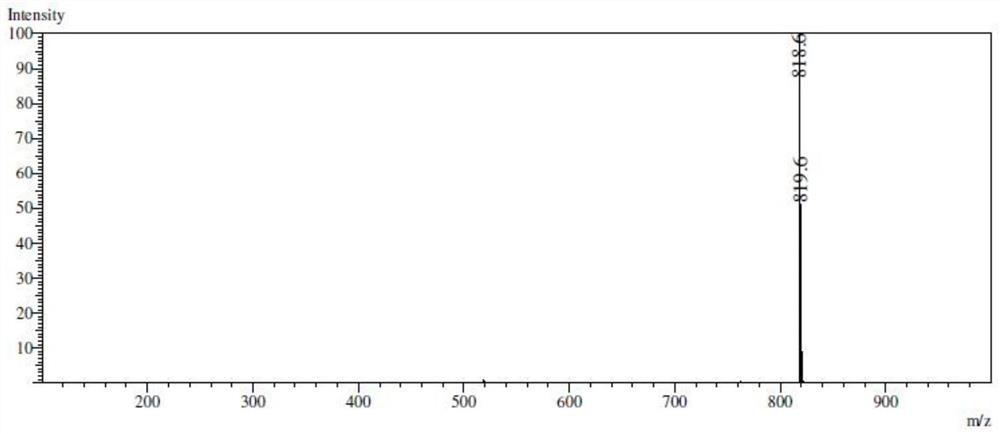
[0077] In a 15.0 mL high temperature pressure-resistant tube, 1.0 mmol of ligand BQTC and 1.0 mmol of zinc (II) chloride solid were weighed, respectively, and 3.0 mL of methanol and 0.5 mL of dichloromethane solution were added, and the coordination reaction was carried out at 45 °C. 72h, the product was washed 3 times with 2.5mL methanol-diethyl ether mixed solution (v:v=1:1), and dried in a vacuum drying oven at 45°C to obtain the yellow target product Zn(BQTC), yield: 50.1% .
Embodiment 3
[0079] In a 15.0 mL high-temperature pressure-resistant tube, weigh 1.0 mmol of ligand BQTC and 1.0 mmol of zinc (II) chloride solid, add 1.0 mL of methanol and 5.5 mL of chloroform solution, and carry out the coordination reaction at 45 °C 12h, the product was washed 3 times with 2.5mL methanol-diethyl ether mixed solution (v:v=1:1) and dried in a vacuum drying oven at 45°C to obtain the yellow target product Zn(BQTC), yield: 70.5 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com