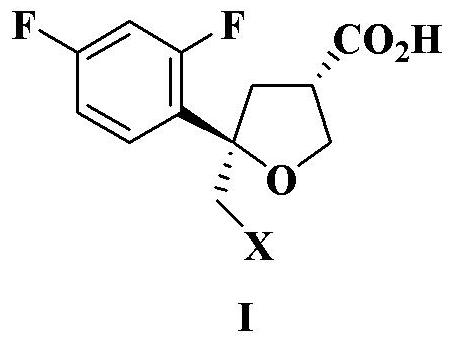
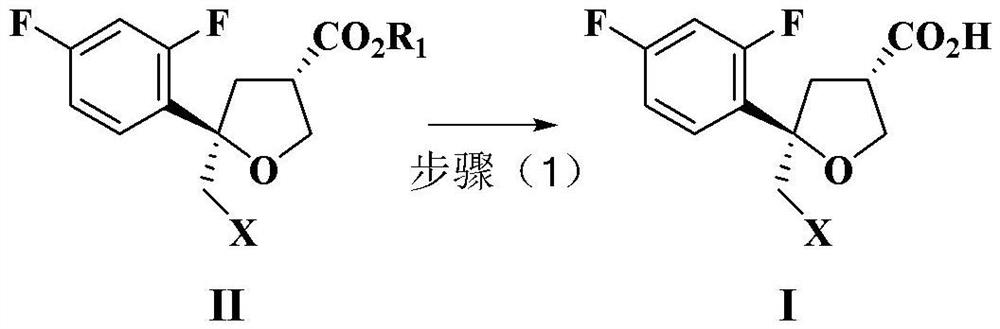
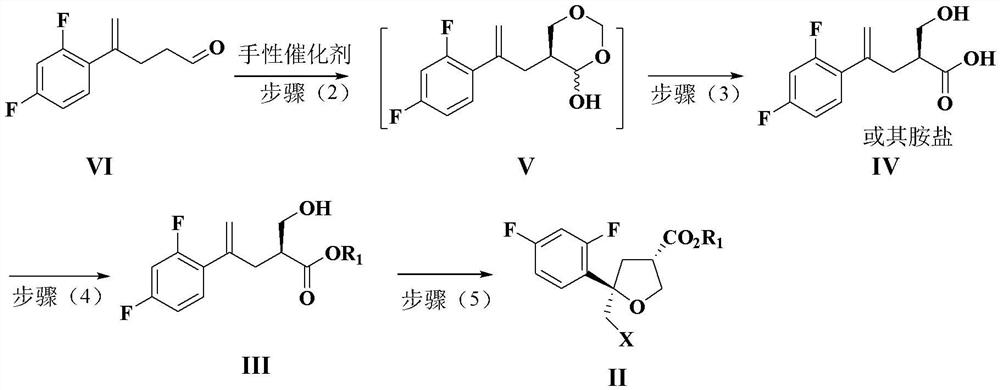
Preparation method of intermediate compound for synthesizing posaconazole and intermediate compound prepared by preparation method
A posaconazole intermediate and compound technology, applied in the field of medicinal chemistry, can solve problems such as not being suitable for large-scale production and affecting yield, and achieve the effects of easy large-scale production, less impurity content, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1: Preparation of compounds of formula VI
[0146]
[0147] Under nitrogen protection, 40 g of the compound of formula X and 200 g of toluene were added to a 500 mL reaction flask, and stirred to dissolve. Use liquid nitrogen to cool down to about -80°C, add 90g of 1.5M toluene solution of diisobutylaluminum hydride (DIBAL-H) dropwise, and keep the reaction for about 2.0h after dropping. Then control the temperature in the range of -5~20℃, slowly add the reaction solution to 400g 3M hydrochloric acid solution for quenching, stir for 3.0h after quenching, let stand for stratification, wash the organic layer twice with 80g×2 water, A toluene solution of the compound of formula VI is obtained. The obtained solution was directly used in the next reaction without concentration.
Embodiment 2
[0148] Example 2: Preparation of Chiral Catalyst (R-PDD-TMS)
[0149]
[0150] Add 40g R-diphenylprolinol (R-PDD), 400mL dichloromethane and 32.2g imidazole to a 500mL reaction flask, stir to dissolve, cool down to 0°C, and dropwise add 50mL trimethylchlorosilane (TMSCl) , after dripping, the temperature was raised to 25 °C for 12 h and the reaction was incubated for 12 h. After the insulation was completed, 1000 mL of methyl tert-butyl ether was added, and after stirring for 30 minutes, the filtrate was filtered. 2 SO 4 It was dried and concentrated under reduced pressure to obtain 50 g of oil. Yield 97%, HPLC purity 98%.
Embodiment 3
[0151] Example 3: Preparation of compounds of formula V
[0152]
[0153] 1.5g of chiral catalyst R-PDD-TMS and 2.0g of KH were added to the toluene solution of the compound of formula VI prepared in Example 1 above. 2 PO 4 and 3.1g K 2 HPO 4 And stir for 10 ~ 15min. Then, 15 g of 37% formaldehyde solution was added, the temperature was raised to an internal temperature of about 20° C., and the reaction was maintained for 12 h. 100 g of water was added to the reaction solution, stirred, and the layers were separated. The aqueous layer was extracted with 50 g of toluene. The aqueous layer was discarded. The organic phases were combined and concentrated to dryness under reduced pressure to obtain 40 g of the compound of formula V. Yield: 88%, HPLC purity: 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com