Lithium ion battery indium niobium oxide negative electrode material and preparation method thereof
A technology of lithium-ion batteries and niobium oxides, applied in the direction of niobium compounds, battery electrodes, chemical instruments and methods, etc., to achieve the effect of improving rate performance, good cycle stability, excellent cycle stability and rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
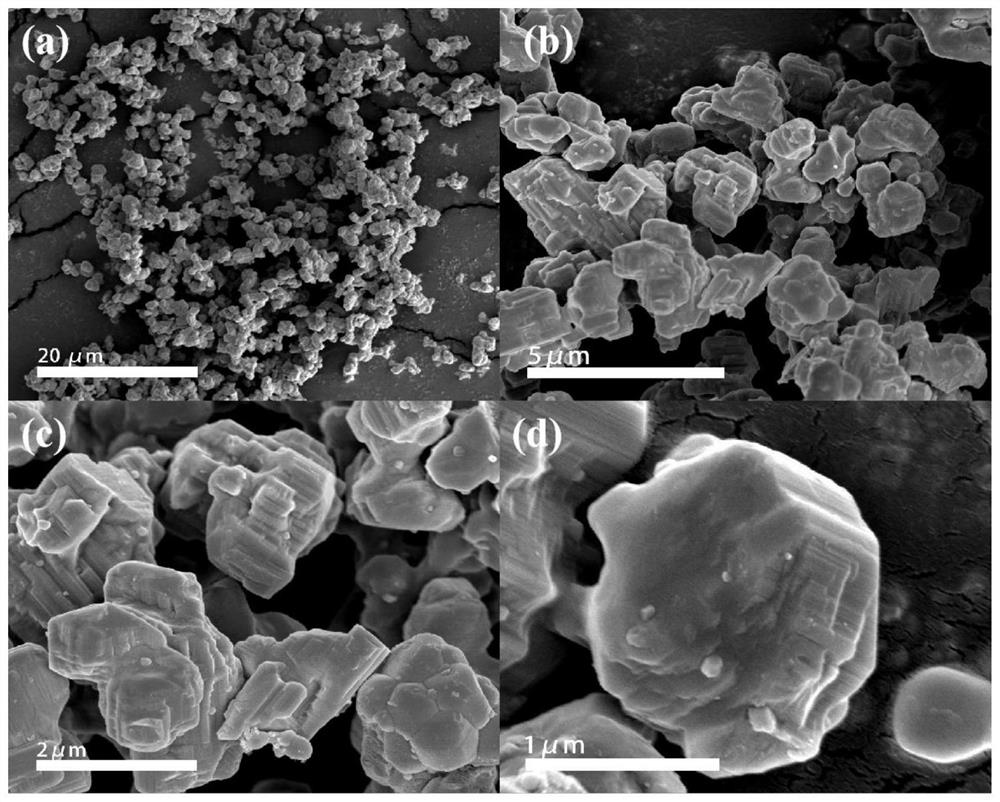
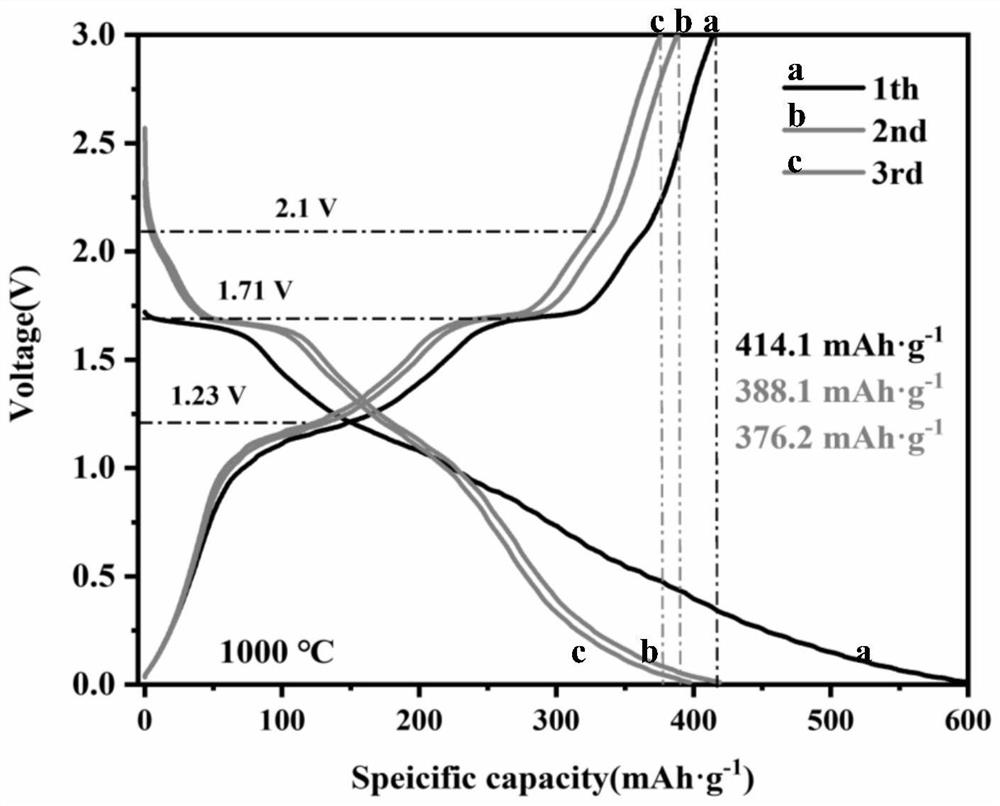
Image
Examples
Embodiment 1
[0026] Stoichiometric ratio In 0.5 Nb 24.5 O 62 Preparation of anode material:
[0027] Using niobium pentachloride and indium trichloride as raw materials, select polyether F127 as additive, control In x Nb 2-x O 5-x In x=0.04, the preparation stoichiometric ratio In 0.5 Nb 24.5 O 62 negative electrode material. Take 0.004 mol of niobium pentachloride, weigh 0.2 g of polyether, and mix them after they are dissolved completely. The mixed solution was put into a high temperature reaction kettle and put into a constant temperature oven for reaction, the reaction temperature was 200°C, and the reaction time was 24h. After the reaction was completed, the precipitate was taken out, washed with water and absolute ethanol for several times, and then placed in an oven to dry. The dried samples were calcined in a tube furnace in an air atmosphere, the calcination temperature was 1000 °C, the calcination time was 6 h, and the heating rate was 4 °C min -1 , after the heat pres...
Embodiment 2
[0029] Different crystal forms of In 0.5 Nb 24.5 O 62 Preparation of anode material:
[0030] Using niobium pentachloride and indium trichloride as raw materials, select polyether F127 as additive, control In x Nb 2-x O 5-x In x=0.04, the preparation stoichiometric ratio In 0.5 Nb 24.5 O 62 negative electrode material. Take 0.004 mol of niobium pentachloride, weigh 0.2 g of polyether, and mix them after they are dissolved completely. The mixed solution was put into a high temperature reaction kettle and put into a constant temperature oven for reaction, the reaction temperature was 200°C, and the reaction time was 24h. After the reaction was completed, the precipitate was taken out, washed with water and absolute ethanol for several times, and then placed in an oven to dry. The dried samples were calcined in a tube furnace in an air atmosphere. The calcination temperature was 900 °C, the calcination time was 6 h, and the heating rate was 4 °C min. -1 , after the hea...
Embodiment 3
[0032] Cation self-doping In 0.5 Nb 24.5 O 62 Preparation of anode material:
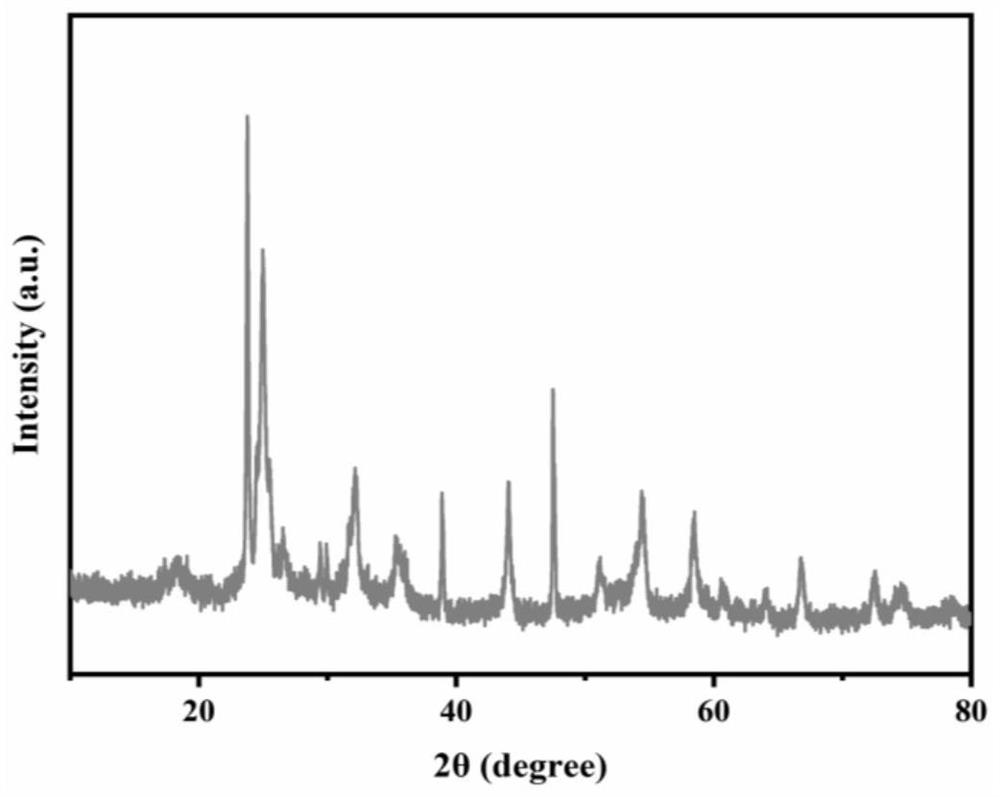
[0033] Using niobium pentachloride and indium trichloride as raw materials, select polyether F127 as additive, control In x Nb 2-x O 5-x In x=0.04, on the basis of Example 1, the excess coefficient of Nb is controlled to be 0.02, and Nb is prepared 5+ Doping In 0.5 Nb 24.5 O 62Anode material, i.e. In 0.5 Nb 24.99 O 63.225 . The rest of the conditions remain the same. The material structure still matches PDF#72-1121 and is monoclinic, as in Figure 5 However, the unit cell parameters and unit cell volume are changed, the localized electronic structure of cations is changed, and the rate performance of the material is improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com